Exploring the Possible Link: Periodontal Disease and Rheumatoid Arthritis
What the evidence says about a potential association between these two common conditions.
The authors have no commercial conflicts of interest to disclose.
This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be
able to:
- Identify the systemic conditions associated with periodontal
disease. - Discuss the evidence surrounding an association between
rheumatoid arthritis and periodontal disease. - Explain the role of infection, citrullinated proteins and
autoimmunity in both diseases. - Note the effects of periodontal treatment on rheumatoid
arthritis.
 In light of their similar epidemiological patterns, environmental factors that influence disease manifestation, and comorbidities with similar systemic conditions for both rheumatoid arthritis and periodontitis, an interrelationship between these two conditions seems plausible.
In light of their similar epidemiological patterns, environmental factors that influence disease manifestation, and comorbidities with similar systemic conditions for both rheumatoid arthritis and periodontitis, an interrelationship between these two conditions seems plausible.
A proposed association between rheumatoid arthritis and periodontitis is not new. As early as 1926, researchers suggested there may be a relationship between arthritis and periodontal disease among individuals with a predisposition to both conditions.6 Subsequent studies have been well documented in narrative reviews.7,8 A systematic review and meta-analysis concluded there is moderate to strong evidence to support an association between rheumatoid arthritis and periodontal disease — and that common risk factors or similar pathologic processes may be responsible for such an association.9
A number of important observations can be made from these studies. First, there is no evidence to support the commonly held idea that rheumatoid arthritis patients have impaired oral hygiene due to reduced dexterity — as both control and rheumatoid arthritis patients have similar plaque and bleeding scores.10,11 Individuals with severe rheumatoid arthritis are more likely to experience advanced periodontitis, and vice versa.10 The role that medications prescribed for rheumatoid arthritis might play in the manifestation of periodontitis is interesting, but may confound the clinical picture. For example, while most individuals with rheumatoid arthritis take medications that reduce inflammation, significant periodontal destruction is still evident in these patients.10 This indicates that periodontitis was most likely developing and not detected prior to the manifestation of rheumatoid arthritis symptoms and subsequent use of medication for the arthritis.12 Thus, determination of the disease duration for both rheumatoid arthritis and periodontal conditions is a critical factor to consider when assessing the relationship between these two conditions. To fully appreciate the associations between periodontitis and rheumatoid arthritis, future studies should aim to document the diseases on the basis of their severity and duration.
PERIODONTAL PATHOGENS AS AN INFECTIVE SOURCE
The claim that rheumatoid arthritis is caused by some form of infectious agent has been postulated for more than 70 years and is still under consideration.13 Numerous studies in a variety of animal models have demonstrated that arthritis can develop following exposure to exogenous infections.13 While extrapolation from animal models to human disease must be done with caution, the findings from these models lend support to a role for various types of infections in genetically susceptible individuals in the development of human rheumatoid arthritis.14
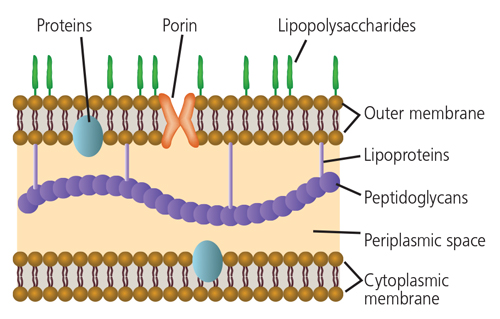
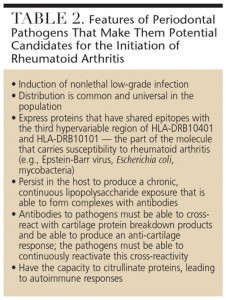
The role of Gram-negative bacteria and their byproducts (particularly lipopolysaccharide) has long been implicated in the development of rheumatoid arthritis (Figure 1).15 Clearly, the subgingival plaque associated with periodontitis (mainly Gram-negative anaerobes) could be a potential source of bacteria and bacterial byproducts for bacterial-induced rheumatoid arthritis.16 Many of the so-called periodontal pathogens exhibit similar characteristics to those microorganisms suspected to induce rheumatoid arthritis in a genetically susceptible host (Table 2). Thus, the possibility that bacteria associated with periodontitis could trigger or exacerbate rheumatoid arthritis in genetically susceptible individuals is biologically possible.
Several studies have demonstrated that patients with rheumatoid arthritis, compared to nonrheumatoid arthritis controls, have elevated antibodies in their serum against several bacteria associated with periodontitis. These includes Porphyromonas gingivalis, Prevotella intermedia, Prevotella melaninogenica, Bacteroides forsythus and Aggregatibacter actinomycetemcomitans.17,18 Furthermore, elevated antibodies and DNA for B. forsythus, P. intermedia, P. gingivalis and Fusobacterium nucleatum have also been identified in synovial fluid.19–21 These findings suggest that periodontal pathogens may translocate from the periodontal tissues to the joints, where they could exacerbate the inflammatory processes occurring within rheumatoid joints.
CITRULLINATED PROTEINS AND AUTOIMMUNITY
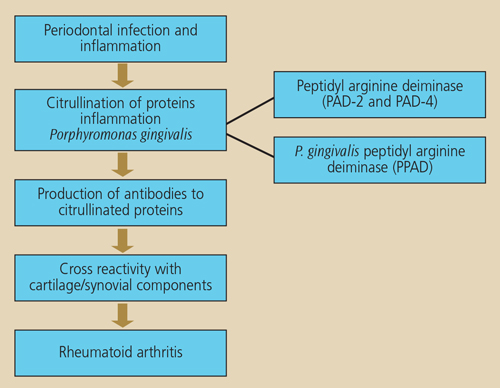
During the course of inflammation, proteins may undergo a process called citrullination that results in a structural change to proteins through conversion of the amino acid arginine to citrulline.22 Citrullination of proteins arises from the action of enzymes, known as peptidyl arginine deiminases (PAD). The change in protein structure as a result of citrullination results in proteins becoming antigenic, which leads to the production of anticitrullinated peptide antibodies. The inflamed periodontium is a potential site where citrullination can occur, and anticitrullinated antibodies can result from this process.23 If such antibodies are produced at sites of inflammation (i.e., during the development of periodontitis) prior to inflammation developing in the joints, the possibility of an enhanced antibody reaction occurring is very high once joint inflammation associated with rheumatoid arthritis develops.24 Importantly, anticitrullinated peptide antibodies have a high predictive value for rheumatoid arthritis onset several years before it is evident clinically, and are also associated with more severe clinical outcomes.25,26
Importantly, P. gingivalis has been reported to be the only bacterium known to produce a PAD enzyme capable of causing protein citrullination.27 Should this bacterial enzyme be able to gain access to the periodontal tissues and be actively involved in protein citrullination in patients with periodontitis, an additional pathway is developed.28 This concept provides a novel link between periodontal infection and the development of rheumatoid arthritis (Figure 2).
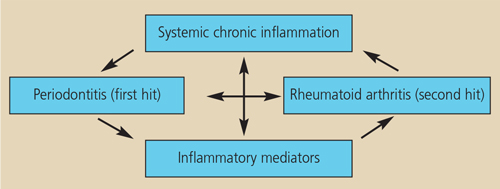
THE “TWO-HIT” MODEL
The primed inflammatory response — or “two-hit” — model has been proposed to explain how periodontitis and rheumatoid arthritis might be interrelated.29 It suggests that a primary hit of chronic inflammation via chronic periodontitis, followed by a secondary inflammatory hit in the joints to induce rheumatoid arthritis can lead to an exacerbated inflammatory response (Figure 3). These heightened responses may lead to increases in numerous mediators of systemic inflammation in the circulation, which may stimulate cells in the joints and periodontium to mediate further tissue destruction.
The most significant clinical relevance for an association between rheumatoid arthritis and periodontitis, however, is the management of patients with both conditions. Even though most clinical protocols for managing rheumatoid arthritis include an assessment for oral pathology and xerostomia (associated with either Sjögren’s disease or medications used in the treatment of rheumatoid arthritis), the signs and symptoms of periodontitis are generally overlooked. This is compounded by the fact that periodontitis, in its early phases, is generally a painless, subtle condition. Its presence often remains undetected until it reaches an advanced stage. If studies can demonstrate that individuals with periodontitis are at higher risk of developing rheumatoid arthritis (or vice versa), then early interventions to assist in controlling the significant tissue destruction noted in both diseases could be implemented.
CONCLUSION
A recent systematic review analyzed the published intervention studies investigating the effect of nonsurgical periodontal treatment on clinical parameters for rheumatoid arthritis in patients experiencing both periodontitis and rheumatoid arthritis.30 This review concluded there is emerging evidence to indicate that periodontal treatment can influence a number of biochemical and clinical markers of rheumatoid arthritis disease activity, including erthryocyte sedimentation rate, levels of tumor necrosis factor-alpha, disease activity score, and anticitrullinated protein antibody levels. The weaknesses of these studies, however, include low subject numbers and short duration (6-month follow-up or less). Therefore, larger population studies with longer follow-up periods are needed to further investigate whether nonsurgical periodontal treatment has any significant effect on clinical indicators of rheumatoid arthritis.
Research demonstrates an association between periodontal disease and rheumatoid arthritis in that they share many of the same manifestations. While causality does not seem to exist between the two diseases, there is a relationship between disease severity and the potential for periodontal infection to either initiate or modulate rheumatoid arthritis. As much remains unknown about this association, more research is needed to explore the possible connection between rheumatoid arthritis and periodontal disease, and what these data may suggest when it comes to prevention and treatment.
References
- Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84(Suppl 4):S8–S19.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25:469–483.
- Horton SC, Walsh CA, Emery P. Established rheumatoid arthritis: rationale for best practice: physicians’ perspective of how to realise tight control in clinical practice. Best Pract Res Clin Rheumatol. 2011;25:509–521.
- Liao KP, Solomon DH. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology. 2013;52:45–52.
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84(Suppl 4):S51–S69.
- Bartold PM, Mariotti AJ Association between periodontitis and rheumatoid arthritis. In: Periodontal Disease and Overall Health: A Clinician’s Guide. RJ Genco, RC Williams Editors. Professional Audience Communications Inc. 2013214-230. Chapter 13, pp 214–230.
- Leech MT, Bartold PM. The association between rheumatoid arthritis and periodontitis. Best Pract Res Clin Rheumatol. 2015;29:189–201.
- Persson RG. Rheumatoid arthritis and periodontitis — inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012;4:1–16.
- Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92:399–408.
- Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–787.
- Mayer Y, Elimelech R, Balbir-Gurman A, Braun-Moscovici Y, Machtei EE. Periodontal condition of patients with autoimmune diseases and the effect of anti-tumor necrosis factor-a therapy. J Periodontol. 2013;84:136–142.
- Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005;76(Suppl 11): 2066–2074.
- Yeoh N, Burton JP, Suppiah P, Reid G, Stebbings S. The role of the microbiome in rheumatic diseases. Curr Rheumatol Rep. 2013;15:314–325.
- Li S, Yu Y, Yue Y, Zhang Z, Su K. Microbial infection and rheumatoid arthritis. J Clin Cell Immunol. 2013;4:4–6.
- Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–578.
- Ogrendik M. Does periodontopathic bacterial infection contribute to the etiopathogenesis of the autoimmune disease rheumatoid arthritis? Discov Med. 2013;13:349–355.
- Scher JU, Bretz WA, Abramson SB. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Curr Opin Rheumatol. 2014;26:424–429.
- Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol. 2014;26:101–107.
- Témoin S, Chakaki A, Askari A, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012;18:117–121.
- Mikuls TR, Thiele GM, Deane KD, et al. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64:3522–3530.
- Reichert S, Haffner M, Keyber G, et al. Detection of oral bacterial DNA in synovial fluid. J Clin Periodontol. 2013;40:591–598.
- Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2013;99:155–163.
- Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013;48:252–261.
- Nandakumar KS. Pathogenic antibody recognition of cartilage. Cell Tissue Res. 2010;339:213–220.
- Moeez S, John P, Bhatti A. Anti-citrullinated protein antibodies: role in pathogenesis of RA and potential as a diagnostic tool. Rheumatol Int. 2013;33:1669–1673.
- Sun J, Zhang Y, Liu L, Liu G. Diagnostic accuracy of combined tests of anti cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2014;32:11–21.
- McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248–3256.
- Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–318.
- Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85:102–105.
- Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):113–122.