 ROBERTODAVID/ISTOCK/GETTY IMAGES PLUS
ROBERTODAVID/ISTOCK/GETTY IMAGES PLUS
Preventing Adverse Events During Sterilization and Disinfection
Implementing proper protocols will help dental teams prevent infection control breaches during sterilization and disinfection procedures — and respond appropriately if a breach occurs.
Cleaning and sterilization or disinfection of reusable patient care instruments and devices are crucial elements of an effective infection control protocol. Rendering dental instruments and devices safe for reuse is a multistep process that requires every step for a given device to be completed in the same sequence each time in order to ensure asepsis. Dental teams should also take steps to confirm — and document — that sterilization methods are successful. As part of efforts to improve outcomes in sterilization and disinfection, medical device manufacturers must now provide validated reprocessing instructions to comply with U.S. Food and Drug Administration requirements.1 Dental health care personnel (DHCP) responsible for reprocessing should carefully read these directions for use (DFU) to ensure they have adequate equipment and materials to process the item.
The varying parameters needed to meet the DFU for each device or instrument may present challenges. For example, similar devices may require different sterilization parameters, such as varying time and temperature; Table 1 highlights how instructions for reprocessing differ among several handpiece manufacturers. In addition, some sterilizers may only offer preprogrammed cycles that do not match the DFU for the device being sterilized. It may not be practical to have multiple cycles parameters in a busy clinical setting where there may only be one autoclave available for all devices. Prior to purchasing instruments or devices, it is important to review the manufacturer’s instructions to ensure the item can be appropriately processed using the available equipment. It may also be necessary to group instruments together that require the same time and temperature, and then sterilize in batches.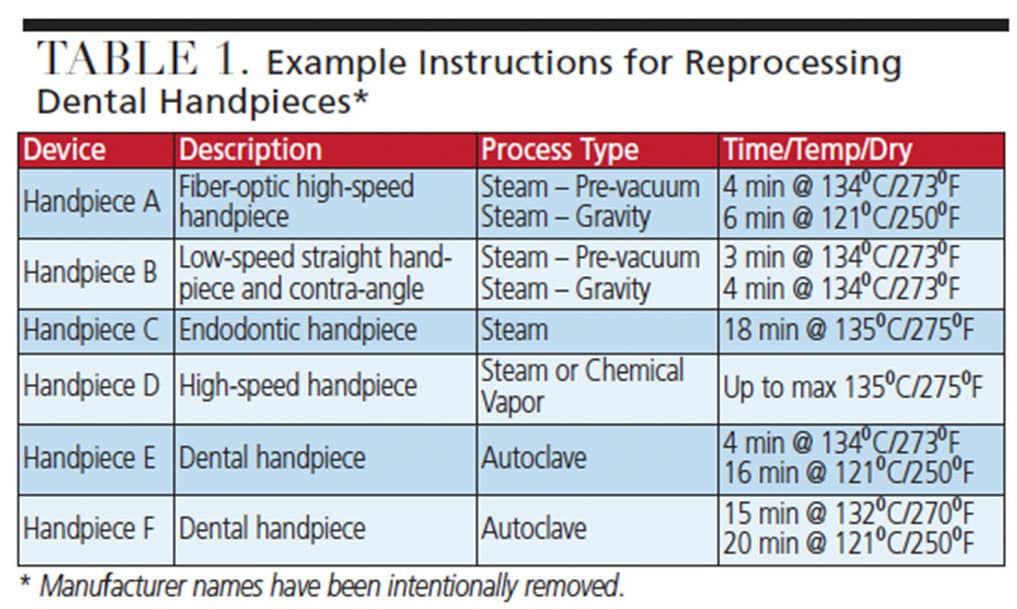
As evidenced by widespread media accounts, failure to follow validated processes and manufacturers’ DFUs for cleaning, sterilization and disinfection may expose patients to infectious diseases.2–6 These breaches in infection control also may impact the licensees in the practice and result in a loss of confidence by patients and the public. Deviating from manufacturers’ DFUs is difficult to defend if questions arise regarding the clinic’s processes. Failure to follow established best practices and manufacturers’ instructions for reprocessing is an infection control breach.

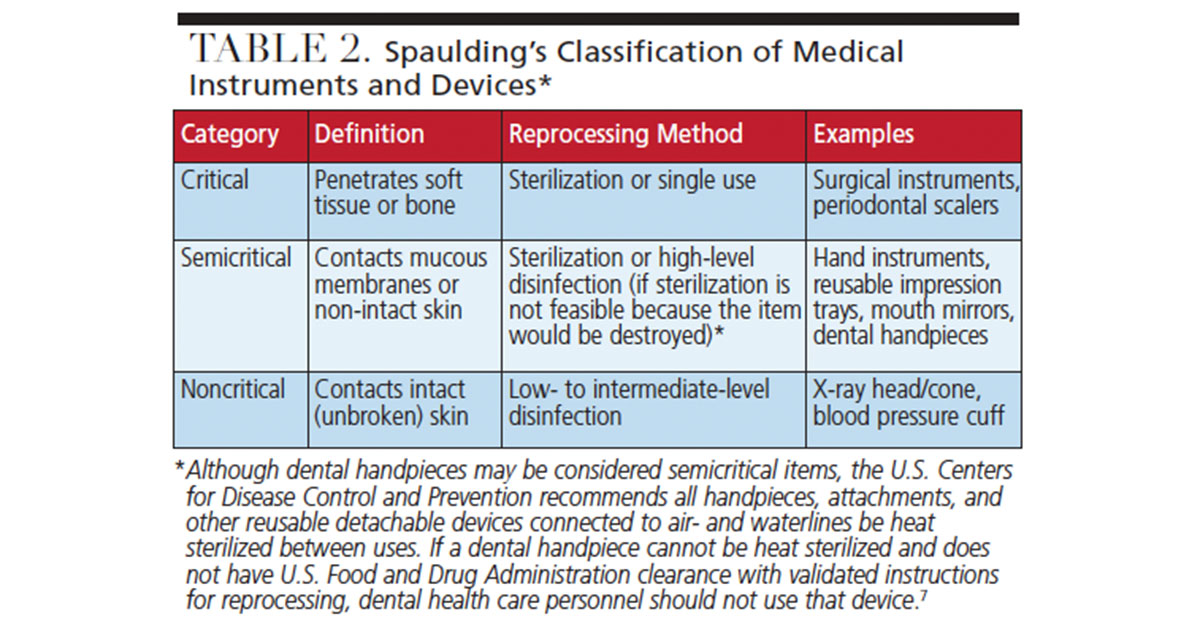
Two common reasons for breaches include inadequate cleaning prior to sterilization of critical or semicritical devices (Table 2), and use of a liquid chemical disinfectant-sterilant instead of autoclaving a device that should be heat sterilized, such as dental handpieces and motors that attach and detach from dental unit air- and waterlines.4–7 The U.S. Centers for Disease Control and Prevention (CDC) unequivocally recommends all dental handpieces and attachments connected to air- or waterlines, including motors, should be heat sterilized between patients and not surface-disinfected. If the handpiece manufacturer does not provide validated reprocessing instructions, that handpiece should not be used.7
STERILIZATION WORKFLOW
An important aspect of instrument reprocessing is to establish a workflow that minimizes the risk for a breach in protocol. The reprocessing area should be separate from patient care areas and designed with adequate space and utilities to allow separation of contaminated and sterile equipment and instruments.8 Regardless of the room layout, the equipment should be arranged to allow the following activities in a one-directional flow in this sequence:
- Collection and sorting of contaminated instruments and equipment
- Cleaning (ultrasonic, washer, or washer/disinfector), including rinsing and drying
- Packaging
- Sterilization
- Storage
For each of the steps listed above, DHCP should consider the personal protective equipment needed, as well as the necessary materials (such as ultrasonic cleaning solutions, chemical indicators, and packaging of appropriate size and weight to keep the items sealed until time of use). Adequate and appropriate storage is an additional consideration. Processed instruments and devices should be stored in an area that is clean, dry and free from contamination, such as a covered or closed cabinet.9

MONITORING STERILIZATION PROCESSES
To ensure the chosen method of sterilization was successful, practices should conduct routine monitoring using a variety of techniques. Team members who perform the monitoring should record the results of each sterilization cycle and maintain records that indicate load parameters. When using steam sterilization, for example, this would include exposure time, pressure and temperature.8–10 Biological monitoring (spore test) results should be recorded and maintained in accordance with best practices and any state licensing board requirements.
Physical Monitoring — Physical or mechanical monitoring involves the use of digital displays, tapes or other recording devices that provide information on exposure time, temperature and pressure during steam sterilization cycles (Figure 1). Before unloading the autoclave, DHCP should review the sterilization parameters to ensure the conditions required for sterilization were met. If the indicators reveal a malfunction during the sterilization process, the contents should not be released for patient use.8–10 Refer to the sterilizer DFUs to determine the appropriate steps to take after an equipment malfunction, and reprocess the items from the suspect load. The Association for the Advancement of Medical Instrumentation, which provides consensus standards for health care facility sterilization, recommends against the use of sterilizers without recording devices (with the exception of sterilizers used with accessory recording devices or printouts).8
Chemical Monitoring — Chemical indicators are designed to assist DHCP in detecting potential sterilization failures that may be related to equipment malfunction or operator error. Even if all parameters for sterilization are met, sterilization may not be achieved if the items were incorrectly loaded in the sterilizer, or if items were placed in incorrect packaging.8 Chemical indicators change color when exposed to high temperatures, and some may react to temperature, pressure and time.8,9 A chemical indicator should be placed on the inside of every pack (Figure 2), and also on the outside if the internal indicator is not visible from the outside of the package (Figure 3).9
While a chemical indicator reacting to heat or time does not prove the items are sterile, it provides assurance the contents were exposed to heat in the sterilizer. Chemical indicators should be checked when removing packs from the sterilizer to ensure they have reacted to the sterilization process. If chemical indicators do not fully change color, items should not be used until they are reprocessed. Checking the chemical indicator before opening a pack of sterile instruments at the point of use will help prevent inadvertent use of unsterilized instruments.9

Biological Monitoring — Biological indicators (BI), also called spore tests, are the most reliable method of monitoring the success of sterilization processes.11 Containing high numbers of bacterial spores that are resistant to the sterilization process, BI testing is designed to confirm sterilization lethality. The CDC recommends use of a BI test at least weekly and with each load of implantable devices.9,11 The BI should be placed in the sterilizer with the load, according to the manufacturer’s DFU. Upon removal, it should either be placed in an incubator with a control from the same lot for in-office testing, or prepared for mailing to a testing facility. If incubating the BI in the dental setting, carefully follow manufacturer’s instructions for incubation time and method of reading test results (usually a change in color and clarity of the liquid suspension).
When using steam sterilization, a single BI failure in loads in which chemical and physical indicators do not indicate failure would not necessarily require recall of the items. Sporadic failures of BIs may occur for reasons that include slight variations in the resistance of the spores, improper use of the sterilizer, and laboratory contamination during culture (which is uncommon with self-contained spore tests).11 The CDC recommends that after a single failed test with steam sterilization, DHCP should remove the autoclave from service, perform another BI test, and review clinical procedures, such as loading and sterilizer settings. If the second BI passes, the autoclave may be returned to service. If the second BI fails, however, the unit should not be used until it is serviced and verified to be functioning properly.11,12
RESPONding TO INFECTION CONTROL BREACHES
The CDC has developed guidance on how to evaluate, manage and respond to a breach in infection control.13 Once a breach in protocol is identified or suspected, immediate action should be taken to ensure the breach is not ongoing. In instrument processing, it may be a single event, such as misidentification of instruments as having been sterilized when they were only cleaned. If this is identified before the instruments can be used on patients, a simple review of processes and protocols should help prevent future occurrences. If the breach is discovered after instruments were used on patients, a more thorough review of the circumstances is needed. The CDC recommends a six-step approach.13
The first step is to identify the nature of the breach, type of procedure, and the bodily fluids or other potentially infected material involved. This includes evaluating current practices and instituting corrective action as soon as possible. The second step is determining the scope of the breach, and identifying the time frame in which it occurred and which patients may have been affected. For example, if the breach involved the lack of sterilization of handpieces per the DFUs, and did not involve other critical or semicritical instruments, the potential patients exposed would be limited to those who had procedures requiring handpieces during that time frame. During Step 2, a literature review should be conducted to assist in evidence-based decision-making and experts should be consulted to assist in assessing risk.
Once the scope and nature of the potential exposure to bodily fluids is determined, Step 3 involves notifying and consulting with key stakeholders. This may include infection control professionals, professional liability insurance carriers, health care providers who may be affected, and licensing agencies or health departments, depending on local regulations. Some of these individuals may be helpful in completing Step 4, which is to perform a qualitative assessment of the breach. The CDC has identified breaches as falling into two broad categories. Category A breaches involve a gross error or demonstrated high-risk practice — for example, reusing needles or syringes on patients. Category B involves a breach with a lower likelihood of blood exposure; an example is using semicritical instruments that have been cleaned ultrasonically, but not heat sterilized. Based on the breach category, Step 5 is to determine if patient notification and testing are warranted. The CDC notes that all Category A breaches warrant notification of patients and testing for bloodborne viruses. For Category B breaches, the decision to notify should be based on information gathered during the assessment and in consultation with key stakeholders. When notifying patients and offering testing, hepatitis B virus, hepatitis C virus, and human immunodeficiency virus should all be included in the testing protocol. There is information in the medical literature regarding how to quantify risk and determine if patient notification is warranted.14
The sixth and final step in the response to a breach or suspected breach is to address communications and logistical issues. Ideally, the stakeholders will reach a consensus regarding notification of the breach. There should be agreement on how the message will be conveyed (e.g., certified mail to patients, phone calls, and/or notification of local press outlets). Once patients are notified of a breach, it should be anticipated that media and legal issues may arise and DHCP managing the breach should be prepared to respond appropriately.
CONCLUSION
Regardless of the method of sterilization and disinfection, errors and breaches in instrument processing may be avoided by implementing recommended policies and procedures. Providing team members who are responsible for instrument processing with adequate training, appropriate equipment, and mechanisms to validate processes (such as physical, chemical and biological monitoring) will help ensure safe care.
Maintaining sterile instruments in their pouches until the time of use also allows DHCP to ensure the packaging is intact and that chemical indicators in or on the pack have changed color. If an error does occur that results in potential blood exposure to patients, rapid response, immediate remediation and good communication may help limit the impact of the breach.
KEY TAKEAWAYS
- In order to ensure asepsis when reprocessing instruments and devices, dental teams must adhere to the manufacturer’s directions for use, and follow every step of the multistep process in the same sequence each time.
- Clinical staff should also take steps to confirm and document that sterilization methods are successful.
- Prior to purchasing instruments or devices, it is important to review the manufacturer’s instructions to ensure the item can be appropriately processed using the available equipment.
- To confirm the chosen method of sterilization was successful, practices should conduct routine monitoring using physical, chemical and biological indicators.
- The U.S. Centers for Disease Control and Prevention recommends the use of a biological indicator test (i.e., spore test) at least weekly and with each load of implantable devices.9,11
- Providing team members who are responsible for instrument processing with adequate training, appropriate equipment, and the monitoring mechanisms to validate processes will help ensure the provision of safe care.
REFERENCES
- U.S. Food and Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Guidance for Industry and Food and Drug Administration Staff. Available at: www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM253010.pdf. Accessed June 26, 2019.
- DOH Investigating York County Dentist for Potential Breaches in Infection Control, Sterilization. Available at: infectioncontroltoday.com/sterile-processing/doh-investigating-york-county-dentist-potential-breaches-infection-control. Accessed June 26, 2019.
- OSAP. Oklahoma Infection Control Breach. Available at: www.osap.org/page/Oklahoma. Accessed June 26, 2019.
- Bean M. Patient Sues New Jersey Clinic Over Sterilization Breach, Rusty Equipment. Available at: beckershospitalreview.com/quality/patient-sues-new-jersey-clinic-over-sterilization-breach-rusty-equipment.html. Accessed June 26, 2019.
- Knowles M. Texas Dental Clinic May Have Exposed 9.5k Patients to Infectious Diseases. Available at: www.beckershospitalreview.com/quality/texas-dental-clinic-may-have-exposed-9-5k-patients-to-infectious-diseases.html. Accessed June 26, 2019.
- Demer L. Bethel Dental Patients Asked to Take Blood Tests After Sterilization Breach. Available at: www.adn.com/alaska-news/rural-alaska/2016/09/30/health-provider-reaching-out-to-bethel-dental-patients-after-sterilization-breach/. Accessed June 26, 2019.
- U.S. Centers for Disease Control and Prevention. Statement on Reprocessing Dental Handpieces. Available at: www.cdc.gov/oralhealth/infectioncontrol/statement-on-reprocessing-dental-handpieces.htm. Accessed June 26, 2019.
- Association for the Advancement of Medical Instrumentation. Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. Available at: http://my.aami.org/aamiresources/previewfiles/1709_ST79Preview.pdf. Accessed June 26, 2019.
- U.S. Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Available at: https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf. Accessed June 26, 2019.
- Seavey R. Troubleshooting failed sterilization loads: Process failures and wet packs/loads. Am J Infect Contr. 2016;44(Suppl 5):29–34.
- U.S. Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities. Available at: www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html. Accessed June 26, 2019.
- U.S. Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-care Settings — 2003. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm. Accessed June 26, 2019.
- U.S. Centers for Disease Control and Prevention. Steps for Evaluating an Infection Control Breach. Available at: www.cdc.gov/hai/outbreaks/steps_for_eval_ic_breach.html. Accessed June 26, 2019.
- Rutala WA, Weber DJ. How to assess risk of disease transmission to patients when there is a failure to follow recommended disinfection and sterilization guidelines. Infect Control Hosp Epidemiol. 2007;28:146–155.
From Decisions in Dentistry. July/August 2019;5(7):52–55.