 EDWARDOLIVE/ISTOCK/GETTY IMAGES PLUS
EDWARDOLIVE/ISTOCK/GETTY IMAGES PLUS
Improve Implant Outcomes With Periodontal Maintenance
Implementing a risk-based, patient-specific periodontal maintenance program will help support long-term implant success.
This course was published in the April 2020 issue and expires April 2023. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain dental implant success rates, the definition of long-term success, and strategies for improving clinical outcomes.
- Describe the incidence of peri-implant mucositis and peri-implantitis, as well as factors that influence outcomes in dental implant therapy.
- List clinical and self-care methodologies that help support implant success.
Although dental implant therapy begins with treatment planning and implant placement, long-term success depends on an effective periodontal maintenance program. When supported by a patient-specific self-care regimen, regular assessments and proper debridement, dental implants have a 90% to 98% success rate.1 Long-term success is defined by osseointegration of the implant to the alveolar bone, full occlusal function without pain, and minimal bone loss.
Clinical outcomes are influenced by three main elements: (1) biological factors, (2) mechanical factors, and (3) self-care.2 In periodontal maintenance, biological factors can be measured through visual assessment for the presence of inflammation in the soft tissues surrounding the implant, and noting radiographic changes in the level of the crestal bone. The stability and integrity of the implant and superstructure will influence the mechanical factors.
According to the American Academy of Implant Dentistry, 3 million Americans have dental implants,3 and the number of patients with implants is growing by 500,000 per year. Of these patients, 80% have experienced peri-implant mucositis and 20% present with some stage of peri-implantitis.4 Per the 2017 classifications of periodontal and peri-implant disease, peri-implant mucositis is defined as an inflammatory lesion of the soft tissue surrounding an implant, with no loss of supporting bone. Left untreated, the peri-mucosal lesion can progress into peri-implantitis, with signs of drainage and progressive bone loss.
TREATMENT PROTOCOL
Each office should develop a written protocol for providers to follow when monitoring implants and treating peri-implant disease. However, dental hygienists, in particular, may find themselves in conflicting situations regarding the integrity of the implant when assessing and scaling the specific site. The differences in opinions on this topic can create confusion. The fear of compromising the integrity of the implant or surrounding tissues can lead to improper monitoring and, ultimately, peri-implant disease. Researchers have confirmed it is safe and essential to probe around implants after the initial adaptation of perimucosal seal and osseointegration has been achieved.5
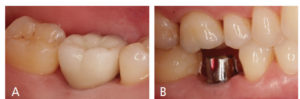
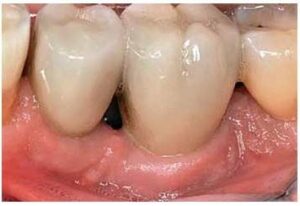
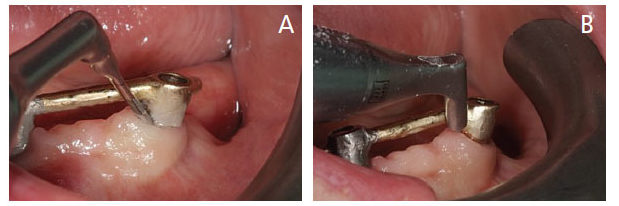
At each periodontal maintenance visit, the provider should visually evaluate the surrounding soft tissues, assessing for peri-implant disease by noting any pain and looking for calculus, cement, mobility and bone level.6 During the visual assessment, the surrounding tissues should appear pink, firm and keratinized (Figures 1A and 1B). Nonkeratinized tissue may lead to peri-implant disease; therefore, the provider should educate the patient on how to achieve keratinized tissue around the implant (Figure 2).7,8 When the clinician assesses for disease, probing and palpation of the supporting alveolar bone must be performed, accompanied by visual evaluation for any signs of infection.
Probe flexibility is important for accuracy and reducing damage to supporting structures. In a study observing clinicians’ probing pressure when administering a periodontal assessment, researchers found the average probing pressure to be 0.15 to 0.20 Newton squared meter (N/m2). Results from this study showed that 0.15 N/m2 might be the pressure threshold to avoid false bleeding on probing.9 Each implant will need a baseline reading following osseointegration to compare at subsequent maintenance appointments. Ideally, implant probing depths should be 2.5 to 5.0 mm or less, and void of bleeding or exudate. The provider should use a bi-digital palpation technique, with one finger on each side of the alveolar bone, and firmly sweep toward the superstructure to evaluate for exudate.6
IMPLANT EVALUATION
Evaluating dental implants for calculus and excess cement is a crucial step in ensuring long-term success. A systematic review of 26 studies totaling 1010 cemented implants, each yielding a disease variance of 1.9% to 75%, found that excess cement was associated with peri-implant disease in 33% to 100% of cases.10 A five-year study conducted in a private practice observed 39 patients with clinical signs of peri-implant disease. The results of the diseased cases indicated 81% were associated with residual cement from placing the superstructure. After removing the excess cement, disease was arrested and the condition of the implants improved in 74% of cases in the short term.11
If screw retention cannot be used to secure the restoration, it is recommended that implant margins be ≤ 1.5 mm subgingival (or clinically visible) to assist in the removal of residual cement.12 Excess cement located in the proximal areas is the most difficult to detect and remove due to deeper implant margins.5 Therefore, early detection and removal of deposits in this crucial area is key to prevent peri-implant disease. Clinicians can use the dental floss technique to detect calculus or excess cement. In addition, radiographs provide an adjunctive method of detecting calculus, cement and open margins.
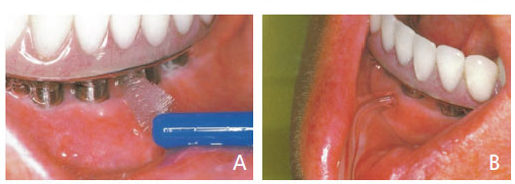
While the dental floss technique is effective in detecting excess cement, research suggests rigorous flossing may not be the best method for self-care. A progressive peri-implant disease study evaluated patients who were compliant with in-office maintenance and presented with excellent self-care. Flap surgery was performed and dental floss remnants were found around the necks of the implants in all subjects. Removing the floss and cleaning the implant resulted in a significant reduction of peri-implant probing depths.13 This study prompted in-vitro testing of the effects of self-care aids on implant surfaces. Researchers discovered that fibers and wax remnants remained on the implant surface after only 10 seconds of rubbing. Conversely, no remnants were detected when interdental brushes were used, suggesting these may be a safer option for self-care (Figures 3A and 3B).13
Unlike natural teeth, implants have no ligaments and, once osseointegration occurs, there should be no mobility. The latter could be due to a loose superstructure, loose or fractured abutment, implant fracture, or complete loss of integration. Clinicians should check for mobility at each visit for both the restoration and implant using the blunt end of a mirror handle to gently push on the superstructure in a buccal-lingual direction. Bubbling of saliva at the gingival margin can be a sign of a loose internal screw, while movement with no bubbling and no pain are due to the external screw or cement not holding.6,14 This lateral movement of a loose restoration will have a clicking or “hard stop,” which is different from the “soft stop” feeling of a loose implant.15
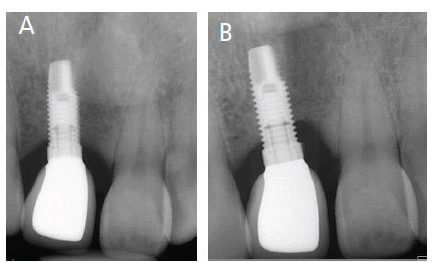
Radiographs are usually taken during the surgical placement of the implant, at the abutment stage, and upon placing the prosthesis. The prosthesis can be placed immediately or after osseointegration occurs. Right-angle periapical radiographs should be taken after placing the definitive restoration, and once a year to compare with the baseline radiographs. In addition, clinicians should evaluate any radiolucency between the implant and crestal bone that might indicate abnormal bone loss (Figures 4A and 4B). After an implant is placed, is was thought that bone remodeling occurs until the biological width is formed, usually resulting in 1 to 1.5 mm of horizontal crestal bone loss.16 However, with new implant surface technology and placement techniques, progressive bone loss is rarely seen.
SELF-CARE AND CLINICAL MAINTENANCE
Self-care is key to a healthy implant and should include both mechanical and chemical methods. Clinicians and patients should work as a team to create and implement the most effective protocol for a given individual. Chemical methods for self-care can include triclosan dentifrices, sodium hypochlorite dilute and chlorhexidine. Research has shown that using a dentifrice containing 0.3% triclosan is more effective in maintaining implant health when compared to a dentifrice without triclosan.17 However, recent controversy has resulted in the ingredient in dentifrices being replaced with stannous fluoride. Although the effect of routine subgingival irrigation is not scientifically significant, a temporary decrease in subgingival bacteria provides an environment conducive to healing.18 Subgingival irrigation can be used during in-office maintenance, as well as self-care.
In a 17-year retrospective analysis, researchers found that implant failure rates can be reduced by 90% with regular periodontal maintenance.19 Although a patient’s risk factors are taken into account when setting recare intervals, a minimum of five- to six-month maintenance interval is needed to reduce the potential for biological complications.8
Subgingival air polishing with glycine powder is a safe and effective mechanical method for biofilm removal around implants (Figures 5A and 5B). Compared to traditional techniques, subgingival air polishing with glycine is faster, less abrasive than prophy pastes, and does not produce heat.20 Considered a low-abrasive method of eliminating biofilm, this modality provides effective surface area coverage without causing alterations to the implant structure.20,21 Researchers have shown that compared to hand instruments, subgingival therapy using a special subgingival nozzle and glycine powder to disrupt biofilm in periodontal pockets, as well as around implants, provides a greater reduction in probing depths and bacterial load.22 Additional studies have noted that compared to other abrasive powders, glycine produces a less rough surface on dental implants.23,24 In another study, glycine air polishing was tested on 180 implants mimicking three levels of peri-implant bone defects; the researchers reported no implant surface damage from the use of glycine powder.25
DEBRIDEMENT AND SUBGINGIVAL IRRIGATION
Mechanical debridement with proper instruments is essential. Available implant instruments include carbon fiber, polytetrafluoroethylene and plastic curets. Compared to titanium curets, researchers have reported carbon-fiber curets cause less damage to implants.6 Ultrasonic devices are another means of mechanical debridement that significantly reduces biofilm and bleeding around implants. Similar to most implant instruments, the tips used on these devices vary according to implant type. Some have a plastic-coated cover to use on implant abutments. However, using these plastic-coated tips when implant threads are exposed is not recommended. In a study evaluating the biological effects of plastic remnants left on implant surfaces, researchers found that 10% to 20% of the 27 surfaces cleaned were covered with plastic remnants.26 The exposed threads can cause the plastic to fragment and embed in the surrounding soft tissues, leading to peri-implant mucositis.6
It is important to note that even though plastic or titanium ultrasonic tips are labeled as a safe method of implant debridement, they should be used with caution due to their ability to generate titanium particles and damage the implant surface.27 These particles have been shown to create a foreign body response, which can lead to bone loss.28
The use of subgingival irrigation as an adjunct to nonsurgical periodontal therapy has been debated for years. Current research offers insufficient support that routine use of subgingival irrigation will increase bone or epithelial migration.18 However, studies show that some subgingival therapeutic agents used as an adjunct to nonsurgical periodontal therapy have a significant effect on the levels of red-complex bacteria. The three main subgingival irrigants used are chlorhexidine, sodium hypochlorite dilute and povidone iodine. The antimicrobial effects of chlorhexidine are weakened when used subgingivally.29 Researchers have demonstrated that when used for subgingival irrigation, 10% povidone iodine significantly reduces periodontal pathogens.30,31 Similarly, sodium hypochlorite dilute is a powerful bactericidal and antiviral solution that has been proven to reduce gingival bleeding and prevent bacterial resistance in periodontal patients.31,32
CONCLUSION
While peri-implant disease is similar to periodontal disease, it should be considered more complex due to the characteristic shift in the microbial flora found in peri-implant conditions.4 To increase long-term implant success, frequent maintenance intervals — including regular radiographic and periodontal assessments — are essential.33,34 The periodontal maintenance schedule must be individualized and based on the patient’s risk factors and oral health status, as well as a meticulous clinical examination.
Mechanical debridement of implants is the foundation of a successful periodontal maintenance program. In conjunction with effective biofilm removal, the use of carbon-fiber scalers to debride the exposed threads of the implant (as needed) will produce an oral environment conducive to implant retention. A significant amount of clinical studies support the use of glycine power to decontaminate and reduce biofilm around dental implants. Cotton pellets or gauze dipped in sterile saline or disinfectant solution, as well as laser-assisted treatments, are effective at removing biofilm and reducing the bacterial load on implant surfaces.35,36 It is important to note a one-size-fits-all approach to implant maintenance will not support long-term success. As part of preventive therapy or when implant disease is present, following an implant maintenance protocol using evidence-based treatment can maintain, or return, the peri-implant mucosa to a healthy state.
REFERENCES
- Bansal P, Dhanya, Bansal P, Singh H, Shanta. Dental implant maintenance — “how to do?” & “what to do” — A review. J Adv Med Dent Sci Res. 2019;7:24–29.
- Pirc M, Dragan IF. The key points of maintenance therapy for dental implants: A literature review. Compend Contin Educ Dent. 2017:38:e5–e8.
- American Academy of Implant Dentistry. Dental implants facts and figures. Available at: https://www.aaid.com/about/Press_Room/Dental_Implants_FAQ.html. Accessed March 13, 2020.
- Valente NA, Andreana S. Peri-implant disease: What we know and what we need to know. J Periodontal Implant Sci. 2016;46:136–151.
- Todescan S, Lanigne S, Kelekis-Cholakis A. Guidance of the maintenance care of dental implants: Clinical review. J Can Dent Assoc. 2012;78:c107.
- Wingrove S. Peri-Implant Therapy for the Dental Hygienist; Clinical Guide to Maintenance and Disease Complications. Iowa: Wiley-Blackwell Inc; 2013.
- Moraschini V, Luz D, Velloso G, Barboza E. Quality assessment of systematic reviews of the significance of keratinized mucosa on implant health. Int J Oral Maxillofac Surg. 2017;46:774–781.
- Monje A, Aranda L, Diaz KT, el al. Impact of maintenance therapy for the prevention of peri-implant diseases: A systematic review and meta-analysis. J Dent Res. 2016;95:372–379.
- Gerber JA, Tan WC, Balmer TE, Salvi GE, Lang NP. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin Oral Implants Res. 2009;20:75–78.
- Staubli N, Walter C, Schmidt JC, Weiger R, Zitzmann NU. Excess cement and the risk of peri-implant disease — a systematic review. Clin Oral Implants Res. 2016;28:1278–1290.
- Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388–1392.
- Levine R, Present S, Wilson TG Jr. Complication with excess cement and dental implants: Diagnosis, recommendations & treatment of 7 clinical cases. Implant Realities. 2014;1:51–59.
- Van Velzen F, Lang N, Schulten E, Bruggenkate C. Dental floss as a possible risk for the development of peri-implant disease: An observational study of 10 cases. Clin Oral Implants Res. 2016;27:618–621.
- Resnik RR. The Dreaded Loose Abutment Screw: Etiology, Management, and Prevention. Available at: https://www.dentistrytoday.com/implants/10398-the-dreaded-loose-abutment-screw-etiology-management-and-prevention. Accessed March 13, 2020.
- Hum S. Managing patients with a loose implant abutment screw. J Can Dent Assoc. 2014;80:e22.
- Alshehri MA. The maintenance of crestal bone around dental implants. Implants. 2011;2:20–24.
- Stewart B, Shibli JA, Araujo M, et al. Effects of a toothpaste containing 0.3% triclosan in the maintenance phase of peri‐implantitis treatment: 2‐year randomized clinical trial. Clin Oral Implants Res. 2018;29:973–985.
- de Freitas CV, Galdez LP, Dias HL, Cirelli JA, Souza EM, da Silva VC. Effect of subgingival irrigation with different substances in the treatment of periodontal disease. A histometric study in rats. J Int Acad Periodontol. 2016;18:2–6.
- Gay IC, Tran DT, Weltman R, et al. Role of supportive maintenance therapy on implant survival: A university-based 17 years’ retrospective analysis. Int J Dent Hygiene. 2016;14:267–271.
- Flemmig TF, Arushanov D, Daubert D, Rothen M, Mueller G, Leroux BG. Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J Periodontol. 2012;83:444–452.
- Petersilka GJ. Subgingival air-polishing in the treatment of periodontal biofilm infections: Subgingival air-polishing. Periodontol 2000. 2011;55:124–142.
- Sahrmann P, Ronay V, Sener B, Jung RE, Attin T, Schmidlin PR. Cleaning potential of glycine air-flow application in an in vitro peri-implantitis model. Clin Oral Implants Res. 2013;24:666–670.
- Biazussi BR, Perrotti V, D’Arcangelo C, et al. Evaluation of the effect of air polishing with different abrasive powders on the roughness of implant abutment surface: an in vitro study. J Oral Implantol. 2019;45:202–206.
- Ronay V, Merlini A, Attin T, Schmidlin PR, Sahrmann P. In vitro cleaning potential of three implant debridement methods. Simulation of the non‐surgical approach. Clin Oral Implants Res. 2017;28:151–155.
- Keim D, Nickles K, Dannewitz B, Ratka C, Eickholz P, Petsos H. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin Oral Implants Res. 2019;30:550–558.
- Yang S, Park J, Ko Y. Use of confocal microscopy for quantification of plastic remnants on rough titanium after instrumentation and evaluation of efficacy of removal. Int J Oral Maxillofac Implants. 2015;30:519–525.
- Harrel SK, Wilson TG Jr, Pandya M, Diekwisch T. Titanium particles generated during ultrasonic scaling of implants. J Periodontol. 2019;90:241–246.
- Suarez-Lopez del Amo F, Garaicoa-Pazmino C, Fretwurst T, Castilho RM, Squarize CH. Dental implants-associated release of titanium particles: A systemic review. Clin Oral Impl Res. 2018;29:1085–1100.
- Abouassi T, Hannig C, Mahncke K, et al. Does human saliva decrease the antimicrobial activity of chlorhexidine against oral bacteria? BMC Res Notes. 2014;7:711.
- Sindhura H, Harsha RH, Shilpa RH. Efficacy of subgingival irrigation with 10% povidone-iodine as an adjunct to scaling and root planing: A clinical and microbiological study. Indian J Dent Res. 2017;28:514–518.
- Perayil J, Menon KS, Biswas R, Fenol A, Vyloppillil R. Comparison of the efficacy of subgingival irrigation with 2% povidone-iodine and tetracycline HCl in subjects with chronic moderate periodontitis: A clinical microbiological study. Dent Res J. 2016;13:98–109.
- Rich SK, Slots J. Sodium hypochlorite (dilute chlorine bleach) oral rinse in patient self-care. J West Soc Periodontol. 2015:63:99–104.
- Wilson TG Jr, Pilar V, Rodrigues DB. Commentary: The case for routine maintenance of dental implants. J Periodontol. 2014;85:657–660.
- Yulan W, Yufeng Z, Miron RJ. Health, maintenance, and recovery of soft tissues around Mimplants. Clin Implant Dent Relat Res. 2016;18:618–634.
- Wheelis SE, Gindri IM, Valderrama P, Wilson TG Jr, Huang J, Rodrigues DC. Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clin Oral Implants Res. 2016;27:329–340.
- Rosen PS, Froum SJ. Peri-implant disease: a primer; etiology, prevention, and treatment. Available at: https://www.aegisdentalnetwork.com/id/2018/09/peri-implant-disease-a-primer. Accessed March 13, 2020.
From Decisions in Dentistry. April 2020;6(4):34–37.




