ALEX-MIT/ISTOCK/GETTY IMAGES PLUS
ALEX-MIT/ISTOCK/GETTY IMAGES PLUS
Treatment of Peri-implant Diseases
These clinical strategies will help in the prevention and management of peri-implant mucositis and peri-implantitis.
This course was published in the June 2020 issue and expires June 2023. Although not in conjunction with this article, the author discloses he has received grants, research support and honorarium from Straumann and Osteogenics Biomedical. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the most recent diagnostic categories proposed to describe the state of dental implant health or disease.
- Discuss etiological factors and interventions associated with peri-implant mucositis and peri-implantitis.
- Describe the role of the titanium oxide layer when attempting bony reattachment in patients diagnosed with peri-implantitis.
Some dental implants fail. While many ideas concerning the genesis of this failure have been proposed, at present, most would argue that bacteria are strongly associated with peri-implant disease and implant loss.1 Numerous secondary factors have also been implicated, including a history of periodontitis, excessive occlusal forces, smoking, diabetes, operator experience, patient adherence (i.e., compliance), and implant type, among others. More recently, the role of foreign bodies has been added to the list of possible etiologic factors.2
While preventing peri-implant disease is a key to success, therapists must be prepared to diagnose and treat conditions that, if left unattended, may lead to implant failure.3 Since dental implants are subject to many of the problems associated with natural teeth, periodic evaluation and maintenance are appropriate. These maintenance visits should include updates of the medical and dental history, as well as dental, periodontal and peri-implant examinations. This data is used to make a diagnosis for each implant. A joint conference sponsored by the American Academy of Periodontology and European Federation of Periodontology proposed the following diagnostic categories: health, peri-implant mucositis and peri-implantitis.1
Peri-implant health was described as an absence of clinical signs of inflammation and bleeding on probing (Table 1). This can exist around implants with normal or reduced bone support. The consensus conference defined peri-implant mucositis as being characterized by bleeding on probing and visual signs of inflammation, which the report states is strongly related to oral biofilm. Progressive bone loss is not seen in this state. It was suggested this process could be reversed with measures aimed at eliminating or reducing the bacterial load on and around the implant. The third category, peri-implantitis, was defined as a plaque-associated pathologic condition characterized by inflammation and progressive loss of supporting bone.
It should be noted the consensus conference emphasized the role of bacterial plaque in the etiology and progression of peri-implant problems, and suggested this process can be eliminated, minimized or possibly reversed by reducing or eliminating biofilm. Little attention was given to foreign bodies. This is in spite of the fact excess cement and titanium particles have been associated with inflammatory peri-implant disease.1,2,4 The possible role of occlusal forces was also discounted as a possible contributor to implant loss, despite emerging evidence to the contrary.5

THERAPY PER DIAGNOSTIC CATEGORY
Peri-implant Health — Because the presence or a history of periodontitis has been strongly linked to peri-implant conditions, patients diagnosed with peri-implant health should be seen based on their periodontal diagnosis. This means individuals diagnosed with implant and periodontal health should be seen at least annually. Those with healthy peri-implant tissues and gingivitis could be seen twice annually, while patients with a diagnosis of periodontitis should be seen more frequently. Active periodontitis should be addressed and appropriate therapy applied, with the goal of stabilizing the periodontium until the periodontal condition is controlled. Studies have found patients who follow suggested recare intervals and maintain periodontal health are less likely to develop peri-implant disease than noncompliant individuals.6,7

Peri-implant Mucositis — This condition occurs frequently8 and is assumed to precede bone loss.1 Therefore, prompt treatment is warranted. Improved oral hygiene, in conjunction with professional therapy, has been strongly related to the clinical reversal of this condition.9 Biofilm removal from the implant surface by the patient can be complicated by surface roughness, as well as the design of the implant and implant prosthesis. Recontouring the prosthesis to allow improved access for oral hygiene is often helpful. Thus, clinicians should reinforce effective self-care in order to reduce the microbial load on and around the implant.
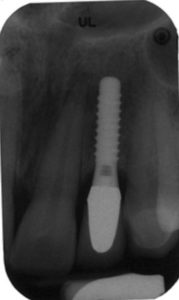
Currently, there are two broad schools of thought as to how this is best achieved. One group aims to reduce biofilm with minimal abrasion of the implant surface. The rationale is that titanium particles have been associated with inflammatory peri-implant disease, and these particles can be generated even with minimal pressure on the implant surface (Figure 1),4,10,11 It should be stated that, at present, concern for generation of these particles is not widely supported; however, the possible significance should not be discounted. If one accepts the possible role of these particles in peri-implant disease, attempts should be made to reduce their production. One technique is a low-abrasion method to reduce the biofilm load using sterile saline on a cotton pellet to gently clean the implant surface. This has been shown to ameliorate or eliminate peri-implant mucositis.12,13 At the same visit, the soft tissue surrounding the implant is gently cleaned with a metallic curet, with its blade turned toward the sulcus wall, away from the implant surface. In some cases, a prescription for a chlorhexidine rinse to be used twice daily is appropriate.
If, in the clinician’s opinion, the generation of titanium particles is not related to the disease process, alternative methods for cleaning the implant surface can be used. Instruments suggested by this second group include manual, sonic and ultrasonic scalers, as well as air powder abrasives. It should be noted recent data has shown these particles are produced by multiple instruments, including stainless steel, titanium and polyetheretherketone plastic tips.14 (See Todescan et al15 for a review of the traditional approach to cleaning the implant surfaces in these cases.)
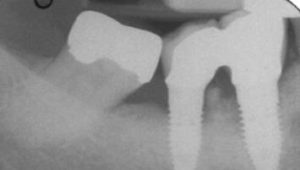
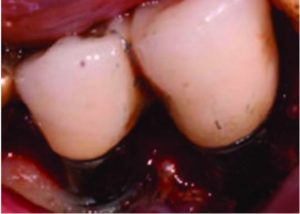
Regardless of the approach taken, a reevaluation at one month is appropriate. Any remaining evidence of peri-implant mucositis may require more aggressive therapy. In cases with cemented restorations, this continued problem has often been found to be related to excess cement particles on the implant surface or in the peri-implant soft tissues (Figure 2, Figure 3 and Figure 4).16 If the clinician suspects this is the genesis and the inflammation process is not controlled by other methods, surgical intervention may be appropriate. This surgical therapy is designed to remove any excess cement on the implant surface, as well as cement and titanium particles in the surrounding soft tissues. If surgical therapy is warranted, the use of the dental videoscope is suggested.17 In these refractory cases, referral to a specialist should be strongly considered.

Peri-implantitis — Although the most effective approach to treating this condition has not been established, based on the clinician’s judgment, surgery may be appropriate in cases of progressive bone loss. Before deciding on the surgical option, data should be gathered, including probing depths, presence or absence of keratinized gingiva, type of prosthesis, right-angle periapical radiographs, history of periodontitis, and dental and medical history. In most cases, a cone beam computed tomogram is valuable. From this data it is possible to determine if the patient is an appropriate candidate for surgery. It will also allow clinicians to determine the approximate percentage of bone in contact with the implant, identify what portion of the rough surface of the implant is suprabony, gain an estimation of the number of bony walls remaining, and determine the need for additional keratinized gingiva.
In most cases, implants with less than 50% remaining bone contact are best removed, and the extraction site grafted and retreated, as indicated. Cases in which implants have adequate remaining supporting bone and where part of the rough surface of the implant is suprabony (e.g., exposed threads) often respond well to the removal of the rough suprabony surface (and any screw threads). This is termed implantoplasty (Figure 5, Figure 6 and Figure 7).18 Various sizes and shapes of 12-fluted finishing burs, followed by the use of appropriate polishing burs, can help remove the rough surface and polish the implant. The soft tissue is placed at an apical position that approximates the bone height. While this has been shown to be successful in some cases, it presents the disadvantages of recession of the peri-implant soft tissues, with more of the implant body exposed — as well as the probability of depositing titanium particles within the surrounding soft tissues. This approach is rarely suggested in areas considered esthetic by the patient. Where bony walls remain, attempts can be made to regenerate bone. Histologic reattachment of bone has been shown in animal studies and been claimed in two human cases.19,20 Once again, there are two general approaches to treating the implant surface when bony reattachment is attempted.
BONY REATTACHMENT
As with peri-implant mucositis, controversy exists on how to appropriately treat peri-implantitis. One camp believes aggressive removal of the biofilm and recontouring the implant surface is most effective.21 Others have demonstrated a less aggressive approach can achieve similar results.12,13,22,23 Bony reattachment (i.e., reosseointegration) seems to depend on the ability of the titanium oxide (TiO) layer to reconstitute once removed. It has been shown in order for bone to attach to the surface of a titanium implant, the surface must be covered by a thin layer of this oxide.24 If one of the goals of treatment is to allow reattachment of bone to the surface, the presence of the TiO layer appears to be important; thus, attempts should be made to avoid further removal of this layer during therapy. That said, this thin layer is easily removed and will not always reestablish itself. If the clinician is confident this layer will reform or feels its reformation is not critical, aggressive removal of the TiO layer should not negatively affect outcomes.
If, on the other hand, the clinician is concerned about the probability of reformation, every effort should be made to preserve the TiO layer. This approach is facilitated by utilizing saline-soaked pellets to reduce implant-bone biofilm,17 as well as use of the dental videoscope — which increases visibility and decreases the probability of damaging the remnants of the oxide layer.
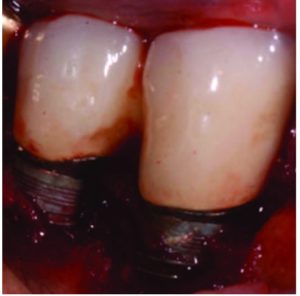
implants seen in Figure 5. This was taken
before implantoplasty.
In most cases with remaining bony walls, once the implant surface has been cleaned, a hard tissue graft is placed. Various materials have been suggested. Many clinicians use human freeze-dried bone (70% mineralized cortical bone, with 30% demineralized bone matrix) mixed with enamel matrix derivative, and covered with a long-acting collagen membrane. Polytetrafluoroethylene sutures are often used and left in place for 30 days. Systemic antibiotics (e.g., amoxicillin 500 mg for use three times per day, starting the day before surgery and continued for seven days) and chlorhexidine rinses (used twice daily for 30 days starting the day after surgery) are prescribed. Follow-ups are scheduled as determined by the response of the tissues and cooperation of the patient.
SUMMARY
Routine maintenance to reduce the biofilm burden on implants and maintain periodontal health around natural teeth is critical to preventing implant failure. These appointments allow clinicians to identify early stages of peri-implant disease and intercede in an appropriate manner. Surgical intervention in refractory cases of peri-implant mucositis may be needed, and is often warranted around implants diagnoses with peri-implantitis.
REFERENCES
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions — introduction and key changes from the 1999 classification. J Periodontol. 2018;45(Suppl 1):S1–S8.
- Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388–1392.
- Monje A, Aranda L, Diaz KT, et al. Impact of maintenance therapy for the prevention of peri-implant diseases: a systematic review and meta-analysis. J Dent Res. 2016;95:372–379.
- Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86:9–15.
- Sridhar S, Wang F, Wilson TG, Palmer K, Valderrama P, Rodrigues DC. The role of bacterial biofilm and mechanical forces in modulating dental implant failures. J Mech Behav Biomed Mater. 2019;92:118–127.
- Rodrigo D, Martin C, Sanz M. Biological complications and peri-implant clinical and radiographic changes at immediately placed dental implants. A prospective 5-year cohort study. Clin Oral Implants Res. 2012;23:1224–1231.
- Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39:173–181.
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254–259.
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–190.
- Eger M, Hiram-Bab S, Liron T, et al. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. 2018;9:2963.
- Mombelli A, Hashim D, Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin Oral Implants Res. 2018;29(Suppl 18):37–53.
- Kolonidis SG, Renvert S, Hammerle CH, Lang NP, Harris D, Claffey N. Osseointegration on implant surfaces previously contaminated with plaque. An experimental study in the dog. Clin Oral Implants Res. 2003;14:373–380.
- Ramesh D, Sridhar, S, Siddiqui D, Valderrama P, Rodrigues D. Detoxification of titanium implant surfaces: evaluation of surface morphology and bone-forming cell compatibility. J Bio-and Tribo-Corrosion. 2017;3:1–13.
- Harrel SK, Wilson TG Jr, Pandya M, Diekwisch TG. Titanium particles generated during ultrasonic scaling of implants. J Periodontol. 2019;90:241–246.
- Todescan S, Lavigne S, Kelekis-Cholakis A. Guidance for the maintenance care of dental implants: clinical review. J Can Dent Assoc. 2012;78:c107.
- Linkevicius T, Puisys A, Vindasiute E, Linkeviciene L, Apse P. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24:1179–1184.
- Wilson TG Jr. A new minimally invasive approach for treating peri-implantitis. Clin Adv Periodontics. 2019;9:59–63.
- Valderrama P, Wilson TG, Jr. Detoxification of implant surfaces affected by peri-implant disease: an overview of surgical methods. Int J Dent. 2013;2013:740680.
- Fletcher P, Deluiz D, Tinoco EM, Ricci JL, Tarnow DP, Tinoco JM. Human histologic evidence of reosseointegration around an implant affected with peri-implantitis following decontamination with sterile saline and antiseptics: a case history report. Int J Periodontics Restorative Dent. 2017;37:499–508.
- Kim S, Hu KS, Jung UW. Reosseointegration after regenerative surgical therapy using a synthetic bone substitute for peri-implantitis: human autopsy study. Int J Periodontics Restorative Dent. 2018;38:585–591.
- Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35:857–863.
- Lang NP, Wilson TG Jr, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implants Res. 2000;11(Suppl 1):146–155.
- Persson LG, Mouhyi J, Berglundh T, Sennerby L, Lindhe J. Carbon dioxide laser and hydrogen peroxide conditioning in the treatment of periimplantitis: an experimental study in the dog. Clin Implant Dent Relat Res. 2004;6:230–238.
- Sul YT. On the bone response to oxidized titanium implants: the role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration [thesis]. Department of Biomaterials/Handicap Research, University of Go, Sweden, 2002.
From Decisions in Dentistry. June 2020;6(6):32–35.