Laser Therapy in Periodontal Treatment
While various laser technologies are available to oral health professionals, the evidence supporting their use in treating periodontal disease is mixed.
The authors have no commercial conflicts of interest to disclose.
This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be able to:
- Identify the types of lasers used in dentistry.
- Discuss the available evidence on the efficacy of laser therapy in dentistry.
- Explain the American Dental Association and American Academy of Periodontology statements on laser use in dentistry.
The use of lasers to treat periodontal disease has become an important topic, and there is ongoing debate regarding the efficacy of lasers in dentistry — and periodontics, in particular. The ability of certain lasers to efficiently cut soft tissue or ablate soft tissue lesions, however, is generally undisputed. This article will explore the types of lasers used by oral health professionals, the evidence-based research regarding laser therapy in the dental setting, and the American Dental Association’s (ADA) and American Academy of Periodontology’s (AAP) recommendations regarding their use in treating periodontal disease.
The lasers with the longest track record in dentistry are the diode, carbon dioxide (CO2), neodymium yttrium aluminum garnet (Nd:YAG), and erbium yttrium aluminum garnet (Er:YAG) lasers. Each type uses different materials in the laser medium to produce varying wavelengths of light. Diode lasers typically operate at a wavelength between 810 nanometers (nm) and 940 nm; CO2 lasers produce a wavelength of approximately 10,600 nm; Nd:YAG lasers operate at a wavelength around 1064 nm; and Er:YAG lasers typically create a wavelength of 2940 nm. Selecting an appropriate laser for the dental practice is based on the hard tissue and soft tissue applications most often performed.
APPLICATIONS FOR LASER TECHNOLOGY
The diode laser is most commonly used by general dentists to displace tissue around crown preparations (in place of packing retraction cord) and for hemostasis and excision of tissue. It is an alternative to electrosurgery and radiosurgery units in many dental practices.3 One advantage is the control the diode laser provides during tissue removal, as it enables precise application of light (heat) to the soft tissue. The resulting penetration is three cell layers to five cell layers thick.3 Diode units are typically less expensive and smaller than CO2 or Nd:YAG models. Diode laser energy can penetrate deeper into the tissue than a CO2 laser, and is attracted to pigmentation (e.g., melanin), which can be advantageous, depending on the procedure. Lacking the power of Nd:YAG and CO2 technologies, however, diode units are less efficient for certain soft tissue applications. Due to its wavelength and target molecules (e.g., melanin), the diode laser is not effective at cutting hard tissue, such as teeth or bone. But, overall, it is a cost-effective option for dentists who need a soft tissue laser for a variety of applications.
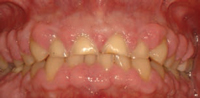
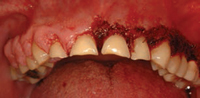
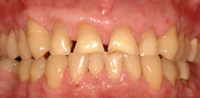
In dental practice, CO2 lasers are primarily utilized for cutting and coagulating soft tissue. This technology has relatively low surface penetration compared to diode and Nd:YAG lasers. Its wavelength is highly absorbed by water, making it a good choice for the vaporization of superficial cells without damaging deeper tissues. The CO2 laser can be used to excise various types of pathology. In addition to removing lesions, it can also be used on a low power setting to remove the surface epithelial layer and ablate various dysplastic lesions. The ability to remove the surface epithelial layer may support the guided tissue regeneration (GTR) process by eliminating or blocking the epithelial downgrowth into the defect following periodontal surgery or scaling and root planing.4,5 Other uses for the CO2 laser include gingivectomy (Figure 1A to Figure 1C), crown lengthening, and implant exposure and uncovery.
A popular soft tissue laser also used for treating periodontal disease, Nd:YAG lasers are able to penetrate more deeply into the tissue (2 mm to 3 mm) than a CO2 laser. One protocol for using Nd:YAG units to treat periodontal defects involves administering local anesthesia, followed by passing the laser fiber-optic tip from the gingival margin to the base of the pocket to remove the diseased pocket epithelium and decontaminate the pocket. The teeth are then aggressively scaled and root planed with ultrasonic and hand instrumentation. An attempt is made to bluntly dissect any remaining periodontal fibers in order to stimulate vascular access to the periodontal wound. A second pass with the laser is performed from the apical extent of the periodontal defect to the gingival margin to form a fibrin coagulum and seal the pocket. No suturing is required. Adjusting any occlusal discrepancies is an important part of the protocol.6
The Er:YAG laser is primarily used in dentistry for hard tissue applications (e.g., calculus removal). This technology is not as popular as the other types because of its limited range of dental applications. In addition to removing calculus from roots of teeth, some clinicians use Er:YAG lasers to remove caries, perform cavity preparations, and etch teeth prior to placing composite restorations. One advantage of using Er:YAG lasers to remove caries lesions is that local anesthesia may not be required. Many clinicians have been reluctant to switch to Er:YAG lasers for cavity preparations because they can’t remove gold, amalgam or ceramic restorations. In addition, the delivery system is more cumbersome than a traditional handpiece, and laser therapy requires more time than conventional techniques.
LASER STUDIES
Beginning in the early 1990s, dentists began extensively evaluating the use of lasers to treat periodontal disease. The first studies primarily looked at the efficacy of CO2 lasers. In 1992, a study by Rossmann et al4 explored the possibility of using a CO2 laser to retard epithelial migration in monkeys through de-epithelialization of the gingival epithelium. On the test side, the oral epithelium was removed by CO2 laser irradiation. Sulcular epithelium was first seen on the test side at 14 days, compared to epithelium becoming immediately present on the control side. For all specimens, there was a trend to less sulcular epithelium and more connective tissue attachment on the test side than on the control side. The authors concluded that, compared to conventional flap surgery, the CO2 laser may be useful in retarding epithelial downgrowth, and thereby enhance new connective tissue attachment. This was the first study to show that a laser could enhance connective tissue attachment when combined with a surgical procedure.4
Gold et al7 examined the effects of Nd:YAG lasers in removing diseased pocket lining epithelium in humans with moderate periodontitis. Twenty-four specimens of gingival tissue from six patients were examined histologically following irradiation with a Nd:YAG laser. Most sections (83%) exhibited complete removal of epithelium. The underlying connective tissue demonstrated no evidence of necrosis or carbonization. Compared to control sites, morphologic features showed minimal change other than removal of pocket lining epithelium. The authors concluded that Nd:YAG lasers can remove pocket lining epithelium in moderately deep pockets without damaging the underlying connective tissue.7
Neill and Mellonig8 combined scaling and root planing with epithelial elimination via the Nd:YAG laser. The authors reported on a double-blind, split-mouth study that involved 10 human subjects randomly assigned to one of three treatments: scaling and root planing alone; Nd:YAG laser plus scaling and root planing; and control only (no scaling and root planing or laser treatment). The researchers evaluated probing depths and clinical attachment levels, as well as several other parameters. They also performed microbial sampling at baseline, one week, one month, and three months post-scaling. The authors concluded that the laser therapy exerted longer lasting effects in altering the subgingival microflora than scaling and root planing alone. The clinical significance of these findings suggested that mechanical scaling and root planing alone may not be the most effective method of treatment in patients infected with Porphyromonas gingivalis or Prevotella intermedia. They also stated there are several areas in nonsurgical therapy in which the adjunctive use of the Nd:YAG laser may be more beneficial than scaling and root planing alone. These include the analgesic effect of the Nd:YAG laser, hemostatic effect, and antibacterial potential of laser energy.8
Israel et al9 published a case series describing an epithelial exclusion technique using CO2 lasers for treating periodontal defects in humans. This paper presented three cases in which vertical bony defects were treated with flap reflection, debridement, grafting with demineralized freeze-dried bone allograft, and de-epithelialization of the outer aspect of the gingiva around the defect site. The patients were seen every 10 days for a repeat of the laser de-epithelialization procedure, for a total of four treatments. In all cases, significant osseous fill was obtained. The authors noted these cases demonstrate the ability to obtain clinical new attachment with bone fill among previously diseased sites. They also concluded that the concept of laser de-epithelialization as an adjunct to regenerative periodontal procedures may show better results than those obtained through conventional osseous grafting alone. In addition, the researchers stated their results appear to be comparable to the results reported for GTR procedures with barrier membranes.9
The effectiveness of Er:YAG lasers in scaling and root planing compared to ultrasonic instrumentation was evaluated by Aoki et al.10 The team studied 53 periodontally involved, extracted human teeth containing a band of subgingival calculus. The teeth were divided randomly into two groups for laser scaling and ultrasonic scaling. The scaled surfaces were examined using histological and scanning electron microscope observations. The authors concluded the Er:YAG laser removed calculus on a level equivalent to that of the ultrasonic scaler, and without major thermal elevation. Macroscopically, the laser-treated root surface was somewhat rougher than the ultrasonically scaled root. In addition, laser scaling efficiency was ranked lower than that of the ultrasonic scaling.10 Additional studies by Frentzen et al11 and Schwarz et al12 support the Aoki study regarding the removal of calculus utilizing Er:YAG lasers.
Gregg and McCarthy13 published a case series that discussed the laser periodontal therapy (LPT) procedure developed specifically for the treatment of moderate to severe periodontitis. The end point of LPT was debridement of inflamed connective tissue, as well as removal of plaque and calculus adherent to the root surface. In addition, the bactericidal effects of the pulsed Nd:YAG laser was designed for reducing microbial pathogens within the periodontal pocket and surrounding tissues. In LPT, the tip of the Nd:YAG laser was used to trough around the tooth. This provided hemorrhage control and relaxation of the tissues for improved access and visualization of the diseased root surface. Following removal of the diseased soft tissue, a piezoelectric scaler, small curets and root files were used to remove root surface accretions.
A second pass with the laser finished debriding the pocket, provided hemostasis, and created a “soft clot.” The tissue was compressed against the root surface to close the pocket and stabilize the fibrin clot. If occlusal trauma was present, it was adjusted with a high-speed handpiece. The authors reported pocket reductions of approximately 70%. They concluded it was possible to achieve similar results using other techniques, but the results in this case series were accomplished using a “closed” noninvasive method. This study helped generate additional interest in treating severe periodontal disease using a nonsurgical approach in combination with laser therapy.13
Yukna et al6 published histologic results in humans following a laser-assisted new attachment procedure (LANAP) for the treatment of periodontal pockets. Six pairs of single-rooted teeth with advanced chronic periodontitis associated with subgingival calculus deposits were treated. A bur notch was placed within the pocket at the clinically and radiographically measured apical extent of the calculus. All teeth were scaled and root planed with ultrasonic and hand instrumentation. One of each pair of teeth received treatment of the inner pocket wall with the Nd:YAG laser to remove the pocket epithelium, and the test pockets were lased a second time to seal the pocket.
After three months, all treated teeth were removed en bloc (meaning the tooth, surrounding soft tissue and bone) for histologic evaluation. The results showed the LANAP-treated teeth exhibited greater probing depth reductions and increased clinical probing attachment level gains than the control teeth. All LANAP-treated specimens showed new cementum and connective tissue attachment in and, occasionally coronally, to the notch, whereas five of the six control teeth had a long-junctional epithelium with no evidence of attachment or regeneration. The authors concluded the LANAP concept may be associated with cementum-mediated new connective tissue attachment and apparent periodontal regeneration of diseased root surfaces.6
In a 15-year follow-up, Crespi et al14 compared the modified Widman flap to coronally advanced flap surgery combined with CO2 laser root conditioning. This is one of the few studies that examined the effects of laser therapy over an extended period (more than five years). Each of the 25 study participants was treated using a split-mouth design. In one quadrant, the teeth received modified Widman flap surgery (control). On the other quadrant, after a full thickness flap was raised, a CO2 laser was used to irradiate the exposed root surfaces and soft tissue. The full-thickness flap was then repositioned coronally and sutured (test). Plaque index and gingival index scores, probing depths and clinical attachment levels were monitored from baseline to the 15-year benchmark. At 15 years post-surgery, the test group experienced greater probing depth reductions and gains in clinical attachment levels than the control sites. The authors concluded that the CO2 laser therapy resulted in significant improvements compared to the modified Widman flap surgery.14
STATEMENTS ON LASERS IN DENTISTRY
The most current ADA Council on Scientific Affairs “Statement on Lasers in Dentistry” reviews sulcular debridement, LANAP, reduction of bacteria levels in periodontal pockets, laser-facilitated wound healing, laser root planing, laser fluorescence, and other hard tissue applications, including endodontics.15 Regarding sulcular debridement, the ADA reports, “The dental literature indicates that when used as an adjunct to meticulous root planing, mechanical or chemical curettage, lasers offer no consistent benefit beyond scaling and root planing alone with respect to gain of the periodontal attachment.”
The ADA also cautions clinicians regarding the effectiveness of the LANAP protocol. “Although the council is optimistic regarding the potential for lasers to enhance effectiveness in treating periodontitis, dentists should note that this study6 provides no more than pilot validation for this treatment concept,” the statement notes. “The council therefore cautions clinicians to weigh the evidence for LANAP when considering the options available for the treatment of periodontal disease.”
In addition, the statement suggests that “lasers, as a group, have inconsistently demonstrated the ability to reduce microorganisms within a periodontal pocket. It appears from the literature that mechanical root debridement remains a priority to attain improvements in clinical attachment levels.” It also notes that the council “considers the application of laser energy purely for the purpose of improved wound healing to be controversial and not well supported by clinical studies.”
The AAP’s “Statement on the Efficacy of Lasers in the Nonsurgical Treatment of Inflammatory Periodontal Disease” is consistent with the ADA’s findings. The AAP states, “There is minimal evidence to support the use of lasers for the purpose of subgingival debridement, either as a monotherapy or adjunctive to scaling and root planing.” Additionally, it found that “current evidence shows lasers, as a group, to be unpredictable and inconsistent in their ability to reduce subgingival microbial loads beyond that achieved by scaling and root planing alone.”16
At present, neither the ADA nor AAP feel there is sufficient clinical evidence to support the use of lasers as a monotherapy for treating periodontal disease. More clinical research is required before lasers become the standard of care for treating periodontal defects and disease. Even though many clinical studies cite improvement in periodontal disease following treatment with either a CO2 or Nd:YAG laser, the sample sizes were small and many lack long-term follow-up. Additional prospective, long-term, randomized controlled clinical trials are required to ascertain the efficacy of treating periodontal disease with laser therapy.
SUMMARY
As presented in the previous studies and meta-analysis performed by the ADA and AAP, lasers have not reached the level of efficacy of other traditional therapies (such as scaling and root planing or GTR) for treating periodontitis. Most studies show that lasers as a monotherapy are not equivalent to existing techniques, and may best be used as an adjunctive aid or in combination with other treatment modalities. More research may lead to lasers becoming a key instrument for the future treatment of periodontal disease.
While the statements by the ADA and AAP caution clinicians about the efficacy of laser therapy in treating periodontal disease, there are many other applications for lasers in dentistry. The ability to precisely cut and coagulate tissues for a variety of soft tissue procedures — including gingivectomies, gingivoplasties, biopsies of soft tissue lesions, ablation of precancerous lesions, crown lengthening, vestibuloplasties and frenectomies — makes the laser an excellent tool for dental professionals. As more research becomes available, it’s likely lasers will play a bigger role in the day-to-day provision of dental care.
References
- Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493–494.
- Goldman L, Gray JA, Goldman J, Goldman B, Meyer R. Effect of laser beam impacts on teeth. J Am Dent Assoc. 1965;70:601–606.
- Christensen GJ. Soft-tissue cutting with laser versus electrosurgery. J Am Dent Assoc. 2008;139:981–984.
- Rossmann J, McQuade M, Turunen DE. Retardation of epithelial migration in monkeys using a carbon dioxide laser: an animal study. J Periodontol. 1992;63:902–907.
- Pope JD, Rossmann JA, Kerns DG, Beach MM, Cipher DJ. Clinic Adv Periodontics. Use of the Carbon Dioxide Laser as an Adjunct to Scaling and Root Planing for Clinical New Attachment: A Case Series Available at: http://www.joponline.org/doi/abs/10.19 02/cap.2013.120061. Accessed November 5, 2015.
- Yukna RA, Carr RL, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27:577–587.
- Gold SI, Vilardi MA. Pulsed laser beam effects on gingiva. J Clin Periodontol. 1994;21:391–396.
- Neill M, Mellonig JT. Clinical efficacy of the Nd:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent. 1997;9(Suppl 6):1–5.
- Israel M, Rossmann JA. An epithelial exclusion technique using the CO2 laser for the treatment of periodontal defects. Compend Contin Educ Dent. 1998;19:86–95.
- Aoki A, Miura M, Akiyama F, et al. In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling. J Periodontol Res. 2000;35:266–277.
- Frentzen M, Braun A, Aniol D. Er:YAG laser scaling of diseased root surfaces. J Periodontol. 2002;73:524–530.
- Schwarz F, Sculean A, Berakdar M, Georg T, Reich E, Becker J. Periodontal treatment with an Er:YAG laser or scaling and root planing. A 2-year follow-up split-mouth study. J Periodontol. 2003;74:590–596.
- Gregg RH 2nd, McCarthy D. Laser periodontal therapy for bone regeneration. Dent Today. 2002;21:54–59.
- Crespi R, Gherlone E, Romanos G. Comparison of modified widman and coronally advanced flap surgery combined with CO2 laser root irradiation in periodontal therapy: a 15-year followup. Int J Periodontics Restorative Dent. 2011;31:641–651.
- American Dental Association. ADA Council on Scientific Affairs: Statement on Lasers in Dentistry. Available at: ada.org/en/about-the-ada/adapositions- policies-andstatements/ statement-on-lasers-indentistry. Accessed November 5, 2015.
- American Academy of Periodontology statement on the efficacy of lasers in the non-surgical treatment of inflammatory periodontal disease. J Periodontol. 2011;82:513–514.




