 VIDI STUDIO/ISTOCK/GETTY IMAGES PLUS
VIDI STUDIO/ISTOCK/GETTY IMAGES PLUS
Avoiding Complications With Mini Implants
This case report of two mini implants used to replace a single mandibular first molar illustrates possible reasons for failure when used in posterior applications.
This course was published in the July 2021 issue and expires July 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain terminology, basic specifications, and reported survival rates associated with implants ≤ 3.5 mm in diameter.
- Discuss applications for these types of dental implants, as well as potential clinical advantages and disadvantages.
- Describe possible reasons for failure of implants ≤ 3.5 mm in diameter.
Advances in dental implants have increased the selection of implant designs, as well as scientific understanding of which design is best suited for a specific clinical condition. Practitioners and patients have also benefited from increased longevity of implant-supported prostheses, better esthetics, and a more sophisticated understanding of the pathogenesis of peri-implant disease. Yet there remains much to learn, including predictable protocols for treating peri-implantitis, risk factors, and a better understanding of the limitations of implants with smaller diameters and lengths, such as mini implants.
The ease of placement of mini implants has increased their use for a variety of clinical situations, including replacement of load-bearing posterior teeth. The standard root form endosseous implant can range in diameter from 3.5 to 7 mm, and from 5 to 16 mm in length. With respect to implants of less than 3.5 mm diameter, there is potential for confusion regarding terminology; for example, mini versus narrow diameter versus small diameter versus reduced diameter. The terms mini, narrow and small diameter implant are commonly used by clinicians and the literature to describe implants with diameters ranging from 1.8 to 3.3 mm.1–4 The term reduced diameter is more general in that it can refer to any implant within the range of 1.8 mm to 3.5 mm diameter, which includes mini and/or narrow diameter implants. More recent literature has begun to use the term reduced diameter.
The length of mini and standard implants is similar. The reduced diameter of the mini implant enables use of less complex surgical techniques, as they can be positioned in areas with decreased bone thickness and without surgical reflection of a mucoperiosteal flap and/or bone augmentation. The quantity and quality of bone available in the jaw typically defines the characteristics (diameter and length) and number of implants. Thus, mini implants are often used to replace mandibular incisors because the alveolar bone is generally thin in the facial-lingual dimension. Another common application is using mini implants for support of overdentures.5,6 However, one might question their use in replacement of posterior teeth — especially molars — that are subject to increased bite pressures during mastication.
In this paper, the authors present a case report of the failure of two mini implants used to replace a single mandibular first molar. The failure resulted in surgical removal of both implants, osseous grafting and, eventually, replacement with a conventional sized implant. In addition to the case report, the discussion will focus on possible reasons for failure of mini implants when used for posterior tooth replacement.
CASE REPORT
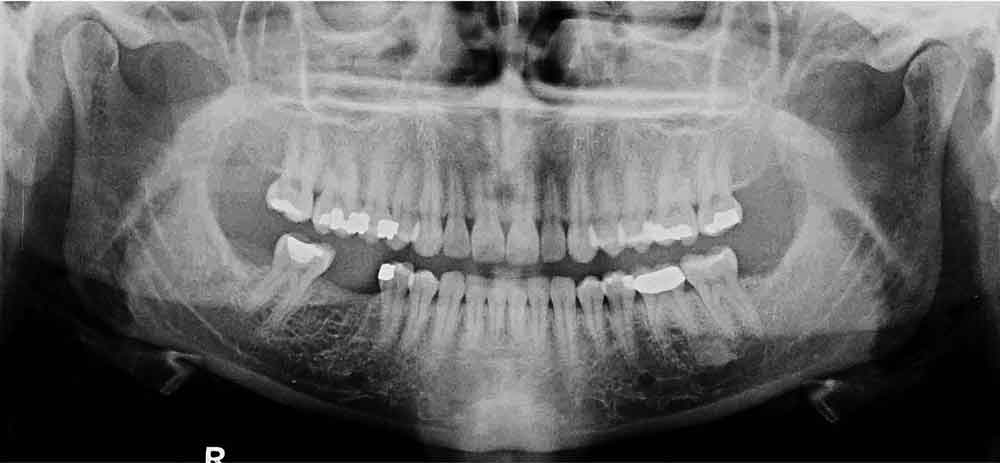
The patient, a 35-year-old female, presented to a general dentist in April 2010 for routine examination and dental prophylaxis. Records from that office indicated no history of periodontal disease, but probing depths were not recorded. Missing teeth included all third molars and the mandibular right first molar (#30; Figure 1). It was noted that tooth #30 had been extracted 10 years prior due to an irreparable tooth fracture. Her medical history was insignificant: no medications, no allergies, no systemic disease, and no history of hospitalization except for childbirth.
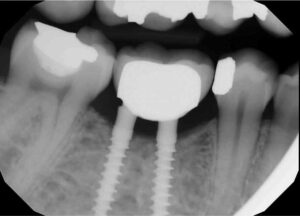
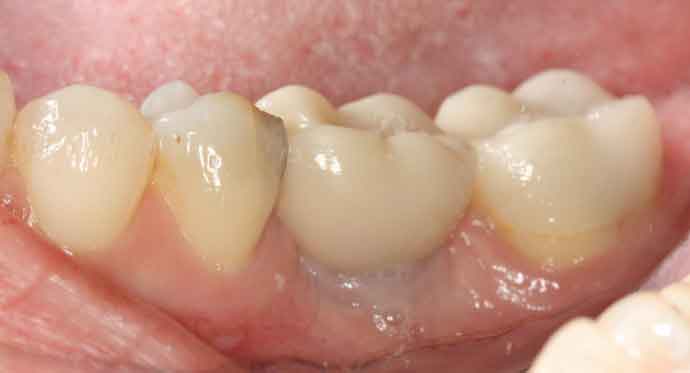
In January 2014, the general dentist suggested replacing edentulous site #30 with an implant. To avoid surgery, mini implants were recommended and placed without mucoperiosteal flap reflection. The implants and single molar restoration at six months post-placement (June 2014) are seen in Figure 2.
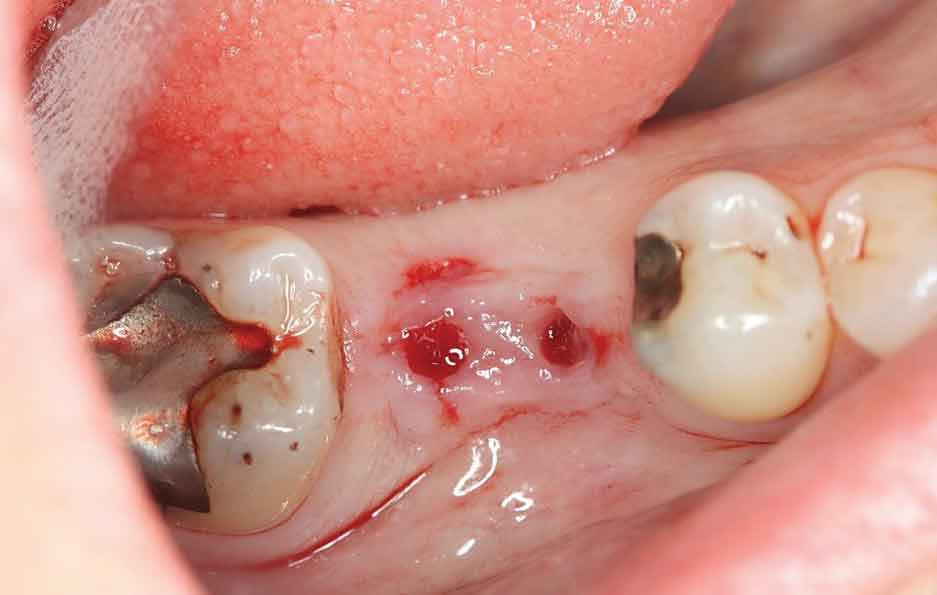
The patient was seen on emergency having been referred to a periodontist (N.E.B.) in May 2015 with a chief complaint of “severe” jaw pain associated with the mini implant site. The examination revealed the implant fixture exhibited Grade II mobility, and the buccal marginal crown extension overlapped the attached gingiva, essentially creating an “engineered” Grade III furcation involvement. While there was little clinical inflammation (Figure 3 and Figure 4), the exam revealed a diminished width of keratinized and attached gingiva at the buccal aspect of the prosthesis; that is, the mucogingival junction was nearly even with the extended crown margin (Figure 3). Based on the patient’s complaint of pain and Grade II prosthesis mobility, a diagnosis of fractured implant(s) was made and it was decided to remove both implants.
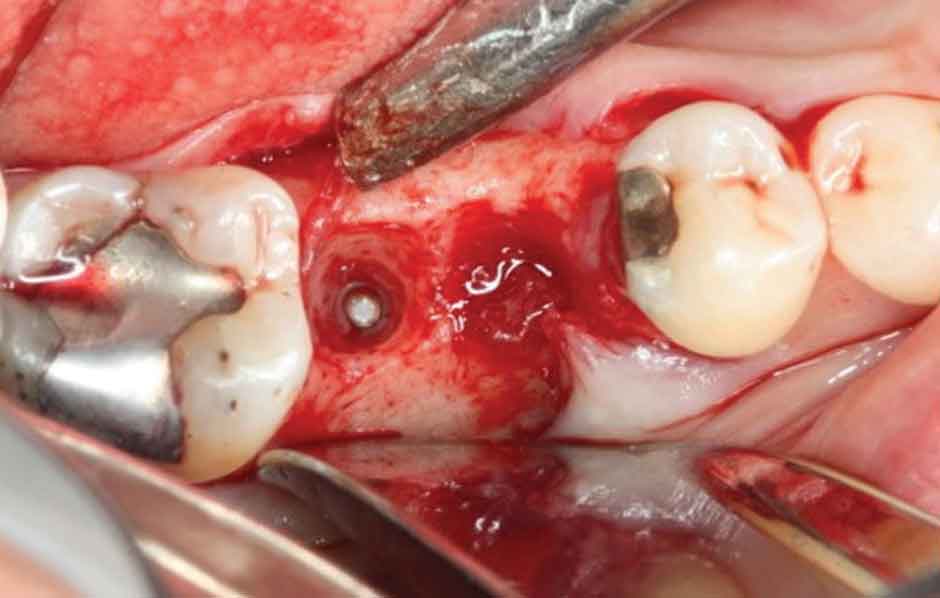
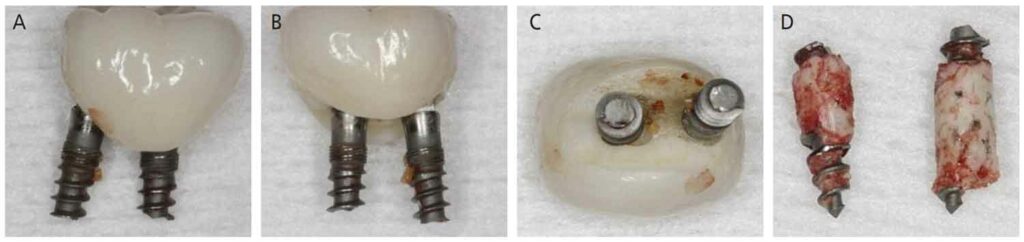
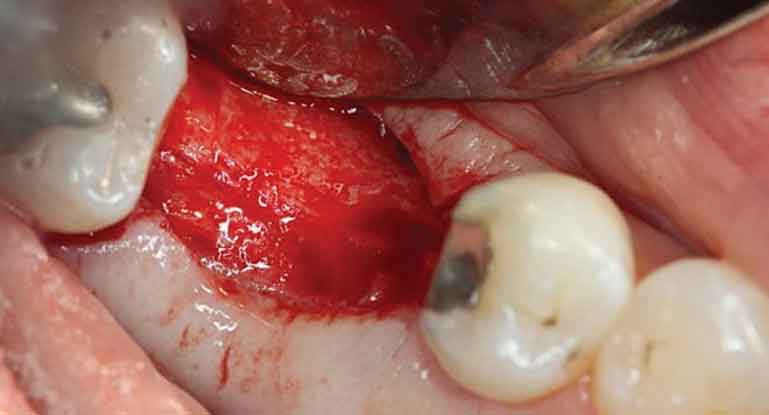
The surgery was accomplished in late May 2015 and consisted of removing both implants and superstructure as a single unit by gentle use of a #217 extraction forceps. Once removed, resorption of the alveolar ridge to the lingual was noted (Figure 4). A full-thickness mucoperiosteal flap was reflected, allowing visualization of the remaining apical portions of both implants at roughly 5 and 6 mm (mesial and distal implants, respectively) below the bony crest (Figure 5). The apical portion of each implant was osseointegrated, necessitating removal by use of a trephine bur (Figures 6A through 6D). Upon retrieval of the fractured implants, it was determined both had dimensions of 2.4×12 mm. The osseous defects were grafted with particulate cancellous bone allograft and covered with pericardium membrane. Next, the surgical site was closed with 4.0 vicryl sutures.
In November 2015, at six months post-osseous grafting, the surgical site was reentered, revealing a well-formed alveolar ridge, with little evidence of resorption in the buccal-lingual dimension (Figure 7). Subsequently, a 4.8×10 mm tissue level, wide neck endosseous implant was positioned, a healing cap placed, and the flap closed with interrupted vicryl sutures.
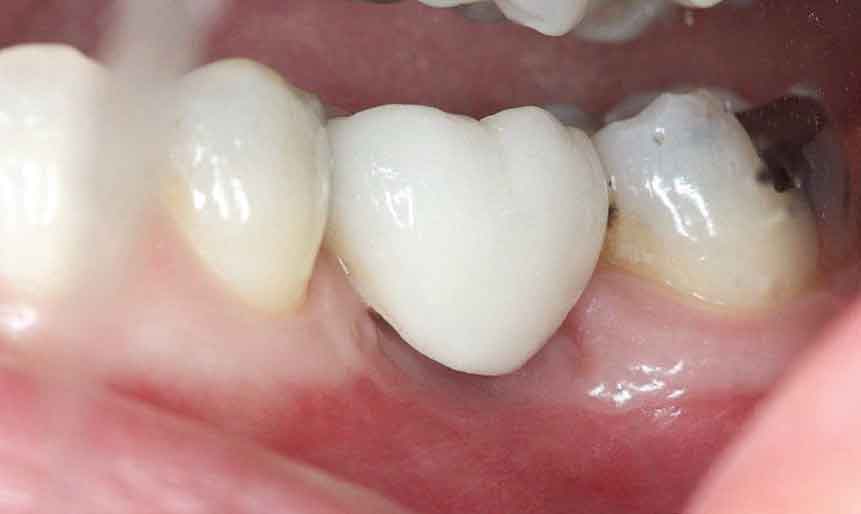
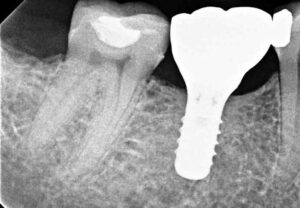
After nine months of healing, the implant was ready for restoration and the patient was referred to her general dentist. A single crown was cemented in August 2016. During a routine follow-up appointment in September 2020, a periapical radiograph showed good bone stabilization and no discernable changes in osseous contour or crestal level since 2016 (Figure 8 and Figure 9).
DISCUSSION
As noted, this is a case report of the failure of two mini implants used to support a single-unit fixed prosthesis replacing a mandibular first molar. While the reason for failure can only be presumed in this case, a logical possibility would be implant fixture fatigue leading to fracture.
Multiple published reports have presented various reasons for failure of smaller diameter implants, including:
- Smoking7,8
- Use of smaller diameter implants8–13
- Posterior localization1,7,11,14
- Prosthetic complications when using narrow diameter implants15
- Placement of implants in the posterior maxilla and atrophic bone1,8
- Risk of implant fracture16,17
- History of periodontal disease18–21
apical portion of the distal implant still within
bone prior to removal using a 3-mm-diameter
trephine bur.
In this case, the patient’s initial complaint that “everything felt loose since they were placed” appears to support the possibility of implant fracture or lack of osseointegration. However, both implants were of equal length and diameter (2.4×12 mm) and, indeed, were fractured at approximately the same level within the supporting alveolar bone. The patient was a nonsmoker and radiographs taken before implant placement showed excellent bone volume and density. Thus, a small implant diameter, the possibility of inordinate torquing force during placement, and a posterior prosthesis with a heavy occlusal load may have interacted to cause failure.
While a search of the literature regarding clinical trials in which mini implants were used to support posterior fixed restorations reveals relatively few papers,1,7,11,22–31 the number is likely inflated due to problematic terminology (e.g., mini versus narrow versus small diameter implant). Further, as previously noted, among these designations, there is an overlap in diameters ranging from 1.8 to 3.3 mm. Consequently, data for implant success is a mixture of mini, narrow and small diameter implant designs.8
Of relevance to the present case is that several reviews addressing success rates of smaller diameter implants have concluded that implant diameter is a significant factor in implant survival.8–13,32,33 Implants with wider diameters achieve better long-term survival rates than those with narrower diameters and corresponding length.8,34 In spite of this conclusion, numerous papers state there is no difference in survival rates of smaller diameter implants versus standard diameters.7,15,23,25–28,30,35 It should be noted that among these latter studies, disparate measures were employed to assess implant performance. Further, in a recent evaluation of clinical trials that reported implant survival rates, Sendyk et al36 noted a significant occurrence of selective outcome reporting. And Klein et al3 in their review of success rates of narrow diameter implants reported that studies were of low quality, and with a high risk of bias.
Also relevant to the current case are the issues of biomechanics and bite force resistance of the mini implant design. Hasan et al9 reviewed the literature related to fatigue life of small diameter and mini implants under normal biting force, and their survival rates. The authors noted that while small diameter and mini implants are reported to have a survival rate over five years of 98.3% to 98.4%, experimental studies conclude that implants with diameters < 3 mm pose an increased risk of fracture. This statement appears to be supported by Flanagan et al17 and the results of their in vitro study of the fatigue life of mini implants. The authors used a fatigue-testing machine designed to apply a cyclic off-axis force. The cyclic load was 300 Newtons (N). The average bite force for an adult ranges between 720 N and 900 N, roughly two to three times that of the experimental load.16 Using this device, implant fracture occurred, on average, after 480,000 cycles. Given the Newton load was roughly one-half to one-third that of average and that humans make tooth contact an average of 700 to 1000 times per day,37,38 this would roughly calculate to 240 to 345 days before implant fracture might occur (using the lower average number of tooth contacts per day). Of course, this is a lab study with many assumptions. Even so, the study constitutes a proof-of-principle with considerations for the practicing clinician.
Lastly, in a review of bite force and its impact on dental implants, Flanagan16 made several observations of importance:
- There is little consistency from patient to patient in the maximum bite force they can generate
- Posterior occlusal bite force is roughly three times that of the anterior
- Implants can be overloaded by a patient’s average bite force
- Bite force should be an important parameter in implant treatment planning
- Clinicians should use qualitative judgment when selecting implant diameter and length, as well as prosthetic design
CONCLUSION
Evolving clinical, biologic and mechanical science is continually challenging the concepts and tenets for successful implant therapy. In spite of innovations in dental implants, clinicians may sometimes exceed the mechanical and biological limits of mini implant design — especially when used for replacing an occlusal load-bearing molar. Caution is needed when considering use of mini implants in situations requiring replacement of a single tooth or multiple posterior teeth. Failure in such scenarios can result in extensive surgery and bone augmentation procedures, thereby negating one of the major reasons for originally choosing the mini implant in lieu of a standard implant.
REFERENCES
- Shatkin TE, Shatkin S, Oppenheimer BD, Oppenheimer AJ. Mini dental implants for long-term fixed and removable prosthetics: a retrospective analysis of 2514 implants placed over a five-year period. Compend Contin Educ Dent. 2007;28:92–99.
- Bidra AS, Almas K. Mini implants for definitive prosthodontic treatment: A systematic review. J Prosthet Dent. 2013;109:156–164.
- Klein MO, Schiegnitz E, Al-Nawas B. Systematic review on success of narrow-diameter dental implants. Int J Oral Maxillofac Implants. 2014;29(Suppl):43–54.
- Flanagan D, Mascolo A. The mini dental implant in fixed and removable prosthetics: A review. J Oral Implantol. 2011;37(Spec Issue 1):123–132.
- Lemos CA, Verri FR, Batista VE, Júnior JF, Mello CC, Pellizzer EP. Complete overdentures retained by mini-implants: a systematic review. J Dent. 2017;57:4–13.
- Jawad S, Clarke PT. Survival of mini dental implants used to retain mandibular complete overdentures: systematic review. Int J Oral Maxillofac Implants. 2019;34:343–356.
- Arisan V, Bölükbaşi N, Ersanli S, Ozdemir T. Evaluation of 316 narrow diameter implants followed for 5‐10 years: a clinical and radiographic retrospective study. Clin Oral Implants Res. 2010;21:296–307.
- Chrcanovic BR, Albrektsson T, Wennerberg A. Reasons for failures of oral implants. J Oral Rehabil. 2014;41:443–476.
- Hasan I, Bourauel C, Mundt T, Stark H, Heinemann F. Biomechanics and load resistance of small-diameter and mini dental implants: A review of literature. Biomed Technol. 2014;59:1–5.
- Pommer B, Mailath-Pokorny H, Haas T, Buseniechner F, Millesi W, Fürhauser T. Extra-short (< 7 mm) and extra-narrow diameter (3.5 mm) implants: a meta-analytic literature review. Euro J Oral Implantol. 2018;11(Suppl 1):S137–S146.
- Scurria MS, Morgan ZV, Guckes AD, Li S, Koch G. Prognostic variables associated with implant failure: a retrospective effectiveness study. Inter J Oral Maxillofac Implants. 1998;13:400–406.
- Higuchi KW, Folmer T, Kultje C. Implant survival rates in partially edentulous patients: a 3-year prospective multicenter study. J Oral Maxillofac Surg. 1995;53:264–268.
- Winkler W, Morris HF, Ochi S. Implant survival to 36 months as related to length and diameter. Ann Periodontol. 2000;5:22–31.
- Moheng P, Feryn JM. Clinical and biologic factors related to oral implant failure: a 2-year follow-up study. Implant Dent. 2005;14:281–288.
- Pieri F, Forlivesi C, Caselli E, Corinaldesi G. Narrow‐ (3.0 mm) versus standard‐diameter (4.0 and 4.5 mm) implants for splinted partial fixed restoration of posterior mandibular and maxillary jaws: A 5‐year retrospective cohort study. J Periodontol. 2017;88:338–347.
- Flanagan D. Bite force and dental implant treatment: A short review. Med Devices (Auckl). 2017;10:141–148.
- Flanagan D, Ilies H, McCullough P, McQuoid S. Measurement of the fatigue life of mini dental implants: A pilot study. J Oral Implantol. 2008;34:7–11.
- Sgolastra S, Petrucci A, Severino M, Gatto R, Monaco A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin Oral Implants Res. 2015;26:e8–e16.
- Sousa V, Mardas N, Farias B, et al. A systematic review of implant outcomes in treated periodontitis patients. Clin Oral Implants Res. 2016;27:787–844.
- Chrcanovic BR, Albrektsson T, Wennerberg A. Periodontally compromised vs. periodontal healthy patients and dental implants: a systemic review and meta-analysis. J Dent. 2014;12:1509–1527.
- Renvert A, Quirynen M. Risk indicators for peri-implantitis. A narrative review. Clin Oral Implants Res. 2015;26(Suppl 11):15–44.
- Anitua E, Errazquin JM, de Pedro J, Barrio P, Begona L, Orive G. Clinical evaluation of tiny 2.5‐ and 3.0‐mm narrow‐diameter implants as definitive implants in different clinical situations: A retrospective cohort study. Eur J Oral Implantol. 2010;3:315–322.
- Anitua E, Saracho J, Begoña L, Alkhraisat MH. Long‐term follow‐up of 2.5‐mm narrow diameter implants supporting a fixed prostheses. Clin Implant Dent Related Res. 2016;18:769–777.
- Degidi M, Nardi D, Piattelli A. Immediate restoration of small‐diameter implants in cases of partial posterior edentulism: a 4‐year case series. J Periodontol. 2009;80:1006–1012.
- de Souza AB, Sukekava F, Tolentino L, Cesar–Neto JB, Garcez–Filho J, Araujo MJ. Narrow‐ and regular‐diameter implants in the posterior region of the jaws to support single crowns: a 3‐year split‐mouth randomized clinical trial. Clin Oral Implants Res. 2017;29(Suppl 3):100–107.
- Herrmann J, Hentschel A, Glauche I, Vollmer A, Schlegel KA, Lutz R. Implant survival and patient satisfaction of reduced diameter implants made from a titanium‐zirconium alloy: A retrospective cohort study with 550 implants in 311 patients. J Craniomaxillofac Surg. 2016;44:1940–1944.
- Lee JS, Kim HM, Kim CS, Choi SH, Chai JK, Jung UW. Long–term retrospective study of narrow implants for fixed dental prostheses. Clin Oral Implants Res. 2013;24:847–852.
- Mazor Z, Lorean A, Mijiritsky E, Levin L. Replacement of a molar with 2 narrow diameter dental implants. Implant Dent. 2012;21:36–38.
- Shatkin TE, Petrotto CA. Mini dental implants: A retrospective analysis of 5640 implants placed over a 12-year period. Compend Contin Educ Dent. 2012;33(Spec 3):2–9.
- Shi JY, Xu FY, Zhuang LG, Gu YX, Qiao SC, Lai HC. Long‐term outcomes of narrow diameter implants in posterior jaws: a retrospective study with at least 8‐year follow‐up. Clin Oral Implants Res. 2018;29:76–81.
- Zweers J, van Doornik A, Hogendorf EA, Quirynen M, Van der Weijden GA. Clinical and radiographic evaluation of narrow‐ vs. regular–diameter dental implants: a 3-year follow‐up. A retrospective study. Clin Oral Implants Res. 2015;26:149–156.
- Song SY, Lee JY, Shin SW. Effect of implant diameter on fatigue strength. Implant Dent. 2017;26:59–65.
- Pérez RA, Gargallo J, Altuna P, Herrero-Climent M, Gil FJ. Fatigue of narrow dental implants: Influence of the hardening method. Materials (Basel). 2020;13:1429.
- Schiegnitz E, Al-Nawas B. Narrow-diameter implants: A systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):21–40.
- Assaf A, Saad M, Daas M, Abdallah J, Abdallah R. Use of narrow-diameter implants in the posterior jaw: a systematic review. Implant Dent. 2015;24:294–306.
- Sendyk DI, Rovai ES, Souza NV, Deboni MC, Pannuti CM. Selective outcome reporting in randomized clinical trials of dental implants. J Clin Periodontol. 2019;46:758–765.
- Miyamoto K, Ishizuka Y, Ueda HM, Saifuddin M, Shikata N, Tanne K. Masseter muscle activity during the whole day in children and young adults. J Oral Rehabil. 1999;26:858–864.
- Miyamoto K, Matsuka Y, Morita M, Yamashita A, Watanabe T. Comparison of biting forces in different age and sex groups: A study of biting efficiency with mobile and non-mobile teeth. J Oral Rehabil. 1999;26:223–227.
From Decisions in Dentistry. July 2021;7(7):31–34.




