 IVAN-BALVAN/ISTOCK/GETTY IMAGES PLUS
IVAN-BALVAN/ISTOCK/GETTY IMAGES PLUS
Ultrasonic Aerosols in the Age of COVID-19
Given concerns over transmission of the SARS-CoV-2 virus, managing aerosols produced during ultrasonic scaling is a key tenet of safe practice.
EDITOR’S NOTE: This is a follow-up to “Dental Aerosols and Spatter Amidst COVID-19,” published in May 2020. While the first article presented an overview of dental aerosols and provided definitions of spray produced during aerosol-generating procedures, this paper — from a noted dental aerosols expert — focuses on the author’s infection prevention recommendations when using ultrasonic scalers.
Oral health professionals are encouraged to consult and remain up to date on recommendations from the U.S. Centers for Disease Control and Prevention, including COVID-19 interim guidance, available at cdc.gov/oralhealth/infectioncontrol/index.html.
Because the SARS-CoV-2 virus that causes COVID-19 can be spread through the air, the scientific world has focused on the presence of the virus in droplets and aerosols. Several studies have reported data that indicate the virus can spread long distances and remain airborne for several hours. These findings have come into question and have not yet been published as peer reviewed research. However, it is widely accepted the virus can be spread through droplets and possibly aerosols. The most studied droplets and aerosols are those produced during medical procedures, such as intubation. Whether the aerosols produced during dental procedures have a large enough viral load to be infectious has yet to be proven. At this point, until there is a study of the infectivity of dental aerosols, they must be treated as potentially infectious and controlled to the greatest extent possible. The ultrasonic scaler is one of the largest producers of spatter droplets and aerosols used in dentistry.1 Figure 1 shows the spatter generated by an ultrasonic scaler when no suction is used to capture the spray. This article will examine what we know about the spray produced by an ultrasonic scaler, and what can be done to make using this key instrument as safe as possible.
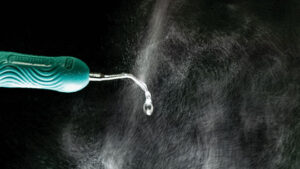
standard amount of coolant water (as recommended by the manufacturer) without
any suction. The material visible in the photograph is termed spatter or droplets. The
aerosol particles are too small to be seen.
The major component of the visible spray created by an ultrasonic scaler is the water used to keep the instrument and tip from overheating, and prevent patient discomfort or damage to the tooth. In most dental offices, the coolant water comes from an independent water supply that is routinely maintained and sanitized. Assuming the dental unit waterline (DUWL) is properly maintained, the coolant spray by itself should not be a danger to patients or practitioners, and can be discounted as a source of infectious material.2
Only when an ultrasonic scaler is used for routine cleaning or root planing does the spray from the ultrasonic become contaminated with bacteria and viruses. It has been shown that even when an ultrasonic scaler is used without any coolant water, potentially infectious material from the operative site will form a spray that can be ejected up to 18 inches from the scaler tip.3 This indicates the operative site (in other words, the patient) is the source of potentially infectious material that is mixed with the spray.4 As this now becomes a potential danger to the dental team and subsequent patients, spatter and aerosols must be carefully managed.
CONTROL CONTAMINATION AT ITS SOURCE
As noted, assuming recommended maintenance of the DUWL is performed and proper asepsis protocols are followed, the chief source of infectious material during ultrasonic scaling is the patient. Based on this, infection control should focus on preventing infectious material from escaping the patient’s mouth and entering the environment of the dental operatory. The use of high level filtration of environmental air, ultraviolet air decontamination, fogging of the operatory after patient treatment, positive air flow with external exhaust, and even the use of an N95 respirator are all aimed at controlling infectious material that has escaped from the patient’s mouth.
Reliance on these external secondary engineering devices as the first line of defense is contrary to the basic principle of infection prevention: Control the infectious material at the source and prevent it from entering the operatory environment. When using an ultrasonic scaler, this means that, to the greatest extent possible, spatter and aerosols must not be allowed to escape the oral cavity. If most of the spatter and aerosols are contained in the mouth, the possibility of the infectious material reaching the operator or settling on environmental surfaces is minimal. Although it may not be possible to totally eliminate spatter and aerosols, if they are reduced by a large percentage, the risk of cross infection will be similarly reduced. All clinical recommendations presented in this article are aimed at reducing the risk of ultrasonic scaling to a level where the routine use of personal protective equipment (PPE) should be adequate to prevent the spread of infection.
PREPROCEDURAL RINSES
The use of preprocedural rinses became routine in many dental offices before the advent of SARS-CoV-2. The purpose of using an antiseptic rinse is to reduce the bioburden of bacteria and viruses in the mouth for the benefit of dental personnel and patients.5 The utilization of a recommended antimicrobial rinse solution should be standard for all patient care. The patient should rinse for 60 seconds upon being seated. This rinse should be repeated after taking a patient history and other preliminary procedures (i.e., just prior to starting treatment).
Rinsing should be repeated several times during treatment. There are several reasons for this. The initial series of rinses will greatly reduce the bioburden, but bacteria and viruses will quickly repopulate the patient’s mouth. One of the greatest sources of contamination will be the cavitation action of the ultrasonic scaler, which will break up and aerosolize plaque and calculus, as well as subgingival crevicular fluid and blood.6 The SARS-CoV-2 virus is known to be harbored in the nasal pharynx (which is, in essence, the back of the mouth), as well as in saliva. Viruses from these sources will be constantly repopulating the oral cavity and become part of the ultrasonic aerosol. Repeated rinses will greatly reduce the population of any potentially infectious materials, thereby reducing the volume of infectious material at the source. In turn, this minimizes the chances of ultrasonic spatter and aerosols containing dangerous levels of pathogens.
CONTROLLING SPATTER AND AEROSOLS AT THE TREATMENT SITE
Yet even if DUWL maintenance and patient rinsing are standard office procedure, it is still advisable to further reduce cross-contamination risks by capturing the spray from the ultrasonic scaler before it exits the mouth. This can be done by placing a large-bore (8 to 10 mm inside diameter) high-volume evacuator (HVE) within 2 cm of the scaler’s working tip. Placing a large-bore HVE at this distance has been shown to reduce the amount of spatter and aerosol and the number of bacteria in the operatory by more than 93%.7–9 Visual observation indicates that placing a HVE further away from the ultrasonic tip will allow a large portion of the spatter and aerosols to escape and enter the operatory.
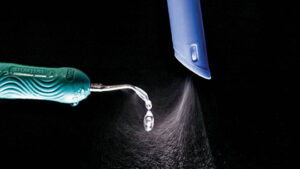
There are two methods of maintaining the HVE close enough to the tip to control spatter and aerosols. The first is the use of a trained assistant during ultrasonic scaling. It must be emphasized the HVE needs to be continuously positioned within 2 cm of the ultrasonic tip. Figure 2 shows a standard handheld HVE tip placed in the correct position relative to the ultrasonic tip. The assistant must be attentive and closely follow the ultrasonic scaler. This can be challenging in certain areas, such as the distal buccal region, where there is limited room and it is difficult to place the HVE in close proximity to the ultrasonic tip. Maintaining the HVE within the necessary distance is extremely difficult using the “two-handed” method in which the operator holds the HVE in one hand and the ultrasonic unit in the other.
A clinical alternative is to utilize an HVE that attaches directly to the ultrasonic scaler and maintains the required close approximation to the ultrasonic tip. A commercially available device that accomplishes this is shown in Figure 3. The original design showed a 93% reduction in spatter and aerosols produced by an ultrasonic scaler,7 and subsequent unpublished tests of a reengineered unit indicate a 97% reduction in spatter.
Other intraoral HVE units, termed “dry field” devices, will undoubtedly reduce the amount of aerosols that escape the mouth, but the HVE suction incorporated in these devices is not placed adequately close (within 2 cm) to the ultrasonic tip; in addition, the HVE does not closely follow the ultrasonic tip to various areas of the oral cavity.

The classic saliva ejector that is frequently used during dental procedures will not adequately remove spatter and aerosols. This is due to the small bore size and its usual placement in the floor of the mouth. While adequate to remove fluids that collect, the saliva ejector is not designed to remove spatter and aerosols and should not be relied on to reduce the spread of infectious material. Figure 4 shows the minimal reduction in ultrasonic spray when a saliva ejector is used for suction.
Some clinicians have advocated having the patient hold a large-bore HVE at the front of the mouth during ultrasonic scaling. While the reduction in aerosols by this method has not been studied and quantified, it is visibly obvious this does not remove a clinically significant amount of spatter and aerosols, but, instead, allows them to escape into the operatory.

SUMMARY
Infection prevention is a matter of reducing exposure to infectious material by preventing viruses and bacteria from escaping their source and entering the environment. In dentistry, this means preventing infectious material from leaving the mouth; and in the case of ultrasonic scaling, this can be accomplished via several steps.
To recap, the clinical steps recommended in this article are to first reduce the chance of infectious material being present in the oral cavity by using multiple antiseptic rinses before and during the procedure. The number of SARS-CoV-2 virus particles and other bioburden should be greatly reduced by this step, but not completely eliminated. The next measure is preventing any remaining infectious material from escaping the mouth. This can be accomplished by the use of a large-bore HVE constantly kept within 2 cm of the ultrasonic tip.
Even with these steps, however, it is possible that a small amount of infectious material may escape into the operatory environment. Thus, the use of the recommended COVID-19 PPE (consisting of a face shield, N95 respirator, and other protection) should be maintained. With this PPE, along with proactive measures to minimize bioburden and contain spatter and aerosols to the oral cavity, potential exposure should be reduced to the point that ultrasonic scaling is safe.
KEY TAKEAWAYS
- Whether dental aerosols have a large enough viral load to be infectious has yet to be proven, but until there is a study of the infectivity of these aerosols, they must be treated as potentially infectious.
- In light of the fact ultrasonic scalers are one of the largest producers of spatter droplets and aerosols,1 this article offers suggestions for making the use of this common instrument as safe as possible.
- Infection control should focus on preventing infectious material from escaping the patient’s mouth and entering the dental operator.
- The use of antiseptic rinses before and during treatment will greatly reduce the patient’s oral bioburden, thus minimizing the risk of infectious aerosols.
- High-volume evacuation (HVE) will also help contain aerosols, but the HVE device must be kept within 2 cm of the ultrasonic scaler’s tip to be effective.
- Even with these measures, providers should use the standard COVID-19 recommended personal protective equipment, including a face shield and N95 respirator.
REFERENCES
- Bently CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125:579–584.
- Murdoch-Kinch CA, Andrews NL, Atwan S, Jude R, Gleason MJ, Molinari JA. Comparison of dental water quality management procedures. J Am Dent Assoc. 1997;128:1235–1243.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc. 1998;129:1241–1249.
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437.
- Fine DH, Korik I, Furgang D, et al. Assessing pre-procedural subgingival irrigation and rinsing with an antiseptic mouthrinse to reduce bacteremia. J Am Dent Assoc.1996;127:641–642,645–646.
- Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by the in vivo use of ultrasonic scalers. J Periodontol. 1998;69:434–438.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67:28–32.
- King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997;68:45–49.
- Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. Gen Dent. 2001;49:648–652.
The author discloses a financial interest in SafetySuction by Quality Aspirators.
From Decisions in Dentistry. July/August 2020;6(7):21-24.


