 GEORGIY DATSENKO / ISTOCK / GETTY IMAGES PLUS
GEORGIY DATSENKO / ISTOCK / GETTY IMAGES PLUS
Mitigating the Risks of Ultrasonic Aerosols
Multilevel infection control steps are needed to provide safe and effective care when using dental ultrasonic units.
This course was published in the August 2022 issue and expires August 2025. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the risks posed by dental aerosols, especially in light of the COVID-19 pandemic.
- Explain strategies that can minimize cross-contamination risks from dental aerosols, particularly while using ultrasonic scalers.
- Discuss the infection prevention advantages of maintaining a high-volume evacuation device within close proximity of the ultrasonic tip.
The COVID-19 pandemic has increased interest in the risks posed by aerosol-generating procedures. Since the emergence of the SARS-CoV-2 pathogen, much of the focus has been on aerosols associated with the hospital care of patients with acute COVID-19. Specifically, many researchers concentrated on the risks of aerosols when removing breathing tubes and clearing the airways of patients with COVID-19 on forced air respirators. This is because patients invariably cough and aerosolize large volumes of material from the trachea and pharynx when the airway tube is removed. Due to the rapid spread of COVID-19 to healthcare workers and confirmed presence of SARS-CoV-2 in this airborne material, the decision was made to label all aerosol-generating procedures as dangerous.
Given this background, all spray and aerosols produced during dental procedures are also considered potentially dangerous. In the initial stages of the pandemic, any procedure that produced aerosols — such as wet drilling and ultrasonic scaling — was banned. Later, severe restrictions on dental aerosol-generating procedures were implemented. As knowledge of COVID-19’s epidemiology has grown and SARS–CoV-2 mutates to strains that cause less severe disease, questions focus on how dangerous dental aerosol-generating procedures actually are to healthcare workers — and what infection control procedures are indicated as we transition into the so-called “COVID normal” world. This article will discuss the current understanding of dental aerosol-generating procedures in general, but will concentrate on the aerosols produced during the use of ultrasonic scalers.
CONTAMINATION FROM ULTRASONIC AEROSOLS
Aerosols produced during ultrasonic scaling procedures are always contaminated and are, therefore, potentially dangerous. There are two sources of aerosol contamination inherent in the use of dental ultrasonic units:1
- The instrument itself, including its source of irrigation/cooling fluid
- Material found in the patient’s mouth
As a source of contamination, the ultrasonic scaler itself does not pose great risk. The handpiece and scaler tip that come into contact with the patient are routinely sterilized between uses. For many modern ultrasonic units, the tubing and connection between the handpiece and instrument console can also be sterilized. When both portions of the ultrasonic instrument are sterile, the instrument itself as a source of infection is eliminated. In some older units, the handpiece and tubing cannot be sterilized; consequently, these devices should be replaced with sterilizable units as soon as possible.
The greater instrument-related infection control concern is the water source for irrigation and cooling of the handpiece and tip. The ideal irrigation source is sterile water, such as a disposable intravenous solution bag or nondisposable sterile solution source that feeds into the ultrasonics’ water system and can be autoclaved between patients. Unfortunately, outside of a few very expensive units primarily designed for ultrasonic surgical cutting, ultrasonic scalers that follow this protocol are difficult to find.
Most ultrasonic scalers either have an independent nonsterile water source or are directly connected to municipal water systems. Both sources can deliver clean — but not sterile — water to the ultrasonic scaler. If these water sources are not scrupulously maintained, however, they can become grossly contaminated, creating a major source of contamination for oral health professionals, as well as patients. Nonsterile water sources should be cleaned and serviced daily following the cleaning product’s manufacturer’s instructions, with a more in-depth cleaning performed at least weekly. In practice, however, these maintenance cleaning steps are frequently not followed, leading to gross contamination of the ultrasonic spray.
PATIENT CONTAMINATION
The greatest source of contamination of the spray and aerosols produced by ultrasonic scalers is from the operating site. In other words, if the instrument and irrigation water source are sterilized and maintained, the spray and aerosol from an ultrasonic scaler is safe. That all changes when the ultrasonic tip is placed in the patient’s mouth and used to remove calculus and biofilm.
Some level of contamination from saliva can be reduced using a preprocedural mouthrinse. However, calculus and biofilm, by their very nature, are filled with bacteria and virus particles (bioburden) that live in the patient’s mouth.2 The protein-binding material in the bioburden is protective against the disinfecting effect of preprocedural mouthrinses such that infectious material remains after rinsing. When calculus and bioburden are removed by the ultrasonic tip, a portion of the bioburden containing bacteria and viruses is mixed with the irrigation solution and becomes airborne. This airborne bioburden — whether the patient has COVID-19 or not — is always a risk for clinicians and potentially other patients and staff in the office.

ARE ULTRASONIC AEROSOLS DANGEROUS?
The aerosols generated by an ultrasonic scaler always present risk. This was recognized by the U.S. Centers for Disease Control and Prevention nearly 20 years ago.3 The use of adequate personal protective equipment (PPE), plus high-volume evacuation (HVE) as an engineering control within the operative site, is required when using an ultrasonic scaler. Since the advent of COVID-19, this is a requirement and not a suggestion.4 The use of HVE is now necessary to meet federal Occupational Safety and Health Administration (OSHA) regulations.5 These guidelines are relatively easy to follow, and, for many, compliance has become routine during ultrasonic usage. The question for most oral health professionals is what equipment is needed. The remainder of this article will discuss the various approaches to compliance as they apply to ultrasonic scalers.
ENVIRONMENTAL DEVICES
Many products are designed to reduce contamination in the operatory atmosphere and entire dental office. These include high-efficiency particulate absorbing (HEPA) filters, various ultraviolet (UV) light systems, external evacuators, and positive flow ventilation. These devices are useful, but do not address the direct reduction of contamination at its source — the treatment site in the patient’s mouth. They instead address any contamination that has escaped from the immediate operating site. These tools, in various forms and in specific circumstances, have been part of hospital infection control for many years. The least expensive is the HEPA filter, but it requires frequent maintenance and filter element replacement to be moderately effective. The most effective and most expensive are “upstairs” UV light units and positive flow ventilation. None of these devices are currently part of OSHA’s requirements for dental offices, but their use may be mandated in the future.

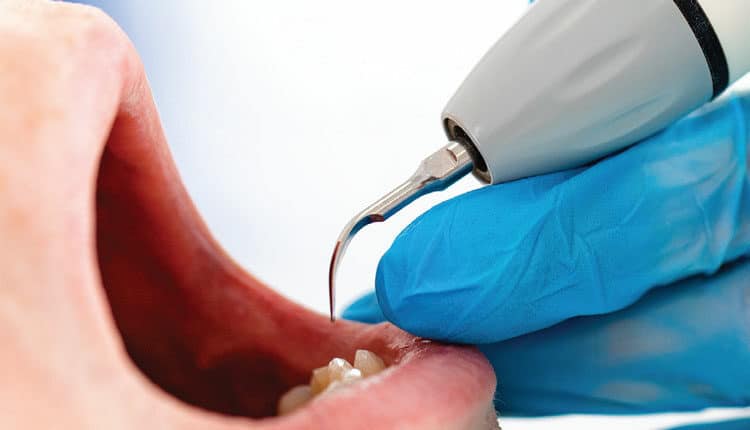
directly to an ultrasonic handle. While the smaller contaminated particles are not visible in this photograph, if the larger and heavier particles are captured, it is highly likely that smaller and lighter particles will also be captured.
PERSONAL PROTECTIVE EQUIPMENT
A large percentage of dental personnel comply with the existing requirements for PPE. Most consider the minimal level of PPE to be gloves, head covering, eye protection, procedure-appropriate masking, and disposable, single patient-use gowns. Initially, N95 filtration level masks were considered mandatory, but recent research has shown that standard surgical masks may be adequate for protection.6 The level of mask filtration necessary remains a question, with the safest approach being the continued use of N95 respirators.
HIGH-VOLUME EVACUATION
The greatest failure in the control of contaminated aerosols is the fact that many dental providers use ultrasonic scalers without adequate HVE. Again, sufficient HVE must be used during ultrasonic scaling. A saliva ejector does not provide HVE, and the use of only a saliva ejector is inadequate for controlling contaminated ultrasonic aerosols. Additionally, this practice does not comply with infection control standards (Figure 1).1
The ideal solution is to have a dental assistant use a large-bore HVE suction tip during ultrasonic scaling. A well-trained dental assistant is expert at keeping the HVE device within 2 cm of the scaling tip. This is the optimal location for removing contaminated aerosols within the patient’s mouth. The goal is to prevent the contamination from escaping into the operatory atmosphere or directly into the operator’s face. Once the contaminated aerosols escape the enclosed environment of the patient’s mouth, they present risk for dental team members and other patients.
Due to economic considerations in clinical practice, most dental hygienists continue to work without an assistant. In this situation, acceptable HVE options for controlling contaminated aerosols include HVE equipment that attaches to the ultrasonic scaler handle (Figure 2) or is inserted into the patient’s mouth. Both approaches fulfill the need to control contaminated aerosols before they escape the oral cavity. Only the HVE device that attaches to the ultrasonic handle has extensive research documenting its effectiveness.7
Some devices are designed to catch the contaminated aerosols just after they escape the patient’s mouth, but prior to the contamination reaching the operator or the operatory environment. These generally have a large-bore HVE positioned on a device that the patient bites on or fits around his or her mouth. These options are often heavy and may need to be frequently repositioned in order to maintain the close approximation to the scaler tip necessary for adequate aerosol control. A lightweight device with flexible edges that surrounds the entire circumference of the mouth and allows easier operator access is shown in Figure 3. There is little research on any of these external devices verifying their ability to reduce contamination. It is always preferable to control the contamination within the mouth rather than after it enters the environment.

COVID-19 PANDEMIC
The greatest recent influence on the delivery of dental care has been the COVID-19 pandemic. Fortunately, the level of COVID-19 cases among dental professionals is low and similar to that of the general population.8,9 The epidemiology of COVID-19 seems to be following a standard curve for infectious diseases. This curve starts with a highly infectious agent — currently SARS-CoV-2 — which causes severe symptoms and death in susceptible and immunocompromised individuals. This was the case in early 2020 when all nonemergency dental procedures were prohibited. As the disease progressed over time, two well-known epidemiologic phenomena occurred:
- The most susceptible patients contracted the disease and either developed natural immunity or succumbed to it
- The virus mutated to a less damaging (i.e., less fatal) form
This has led to the current pandemic situation, which some call COVID normal. This means that COVID-19, like influenza, will remain in circulation. Most individuals will either have natural or vaccinated immunity, which will diminish symptom severity and disease fatality, but not stop transmission of the disease.
CONCLUSION
While COVID normal will alleviate much of the inherent fear felt by healthcare workers early in the disease cycle, the danger of becoming infected remains, and even a mild case of COVID-19 — like the flu — is to be avoided. This means the aforementioned multilevel infection control steps should be considered routine for all dental care. Additionally, these steps are vital during the use of an ultrasonic scaler due to the fact it produces copious amounts of contaminated aerosols that pose risk to the dental team and patients.
The use of HVE during ultrasonic scaling that is effective for “in-the-mouth” contaminated aerosol reduction and strict compliance with PPE requirements are mandatory. Inadequate measures, such as depending on a saliva ejector, must be avoided. Devices and protocols that enable oral health professionals to protect themselves and their patients from contaminated aerosols should be implemented as a routine part of care.
KEY TAKEAWAYS
- All spray and aerosols produced during dental procedures are considered potentially dangerous.
- Sources of aerosol contamination inherent in the use of dental ultrasonic units involve the instrument itself (including its source of irrigation/cooling fluid) and material found in the patient’s mouth.
- Most ultrasonic scalers either have an independent nonsterile water source or are directly connected to municipal water systems. Both sources can deliver clean — but not sterile — water to the ultrasonic scaler.
- If these water sources are not scrupulously maintained, however, they can become grossly contaminated, creating a major source of contamination for oral health professionals, as well as patients.
- Nonsterile water sources should be cleaned and serviced daily, with a more in-depth cleaning performed at least weekly.
- The greatest source of contamination of the spray and aerosols produced by ultrasonic scalers is from the operating site.
- Contamination levels from saliva can be reduced by using a preprocedural mouthrinse.
- The utilization of adequate personal protective equipment, plus high-volume evacuation (HVE) within the operative site, is required when using an ultrasonic scaler.
- Other products or controls designed to reduce contamination in the operatory atmosphere and entire dental office include high-efficiency particulate absorbing filters, ultraviolet light systems, external evacuators, and positive flow ventilation.
- The greatest failure in the control of contaminated aerosols is the fact that many operators use ultrasonic scalers without adequate HVE.
- Keeping the HVE device within 2 cm of the scaling tip is considered the optimal location for removing contaminated aerosols within the patient’s mouth. The goal is to prevent the contamination from escaping into the operatory atmosphere in the first place.
REFERENCES
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437.
- Natto ZS, Afeef M, Bakhrebah MA, et al. Can periodontal pockets and caries lesions act as reservoirs for coronavirus? Mol Oral Microbiol. 2022;37:77–80.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings — 2003. MMWR Recomm Rep. 2003;52(RR-17):1–61.
- U.S. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronvirus Disease 2019 (COVID-19) Pandemic. Available at: https://www.cdc.gov/coronavirus/떓-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Fdental-settings.html. Accessed June 23, 2022.
- U.S. Department of Labor. COVID-19 Control and Prevention, Dentistry Workers and Employers. Available at: https://www.osha.gov/coronavirus/control-prevention/dentistry. Accessed June 23, 2022.
- Infectious Diseases Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients With Suspected or Known COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention. Accessed June 23, 2022.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67:28–32.
- Estrich CG, Gurenlian JR, Battrell A, et al. Infection prevention and control practices of dental hygienists in the United States during the COVID-19 pandemic: a longitudinal study. J Dent Hyg. 2022;96:17–26.
- Madathil S, Siqueira WL, Marin LM, et al. The incidence of COVID-19 among dentists practicing in the community in Canada: A prospective cohort study over a 6-month period. J Am Dent Assoc. 2022;153:450–459.
From Decisions in Dentistry. August 2022;8(8)26-29.




