FDA Takes Action Against Teething Products
The U.S. Food and Drug Administration (FDA) has urged manufacturers of over-the-counter benzocaine products to stop marketing them for treating teething pain in infants and children under the age of 2.
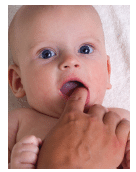
The U.S. Food and Drug Administration (FDA) has urged manufacturers of over-the-counter benzocaine products to stop marketing them for treating teething pain in infants and children under the age of 2. The FDA warns the products carry serious risks for this population, and provide little to no benefits for treating teething pain.
Benzocaine is marketed to help relieve pain from a variety of conditions, including teething, sore throat and oral irritation, and these products are sold as gels, sprays, ointments, solutions and lozenges. However, benzocaine can cause methemoglobinemia, which can be life threatening and result in death. The FDA says it will initiate regulatory action to remove these products from the market if companies do not comply. It has also requested that manufacturers add warnings to all other benzocaine oral health products to inform consumers about what it describes as “serious risks.”
From Decisions in Dentistry. July 2018;4(7):6.


