
Management Strategies for Dental Unit Waterlines
The following methods for controlling and monitoring biofilm in dental water supplies will help safeguard patients and practitioners.
PURCHASE COURSE
This course was published in the April 2017 issue and expires April 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the effects of biofilm formation in dental unit waterlines.
- Identify the infections associated with dental treatment water.
- List the water quality standards for dental offices.
- Explain procedures for effectively managing dental treatment water quality.
Essential to practice, dental unit water is used for a variety of functions, ranging from irrigating surgical sites to providing lavage during restorative procedures. More than five decades have passed since the scientific literature first reported that tubing delivering water to dental devices has a strong tendency to develop biofilm, resulting in patient exposure to water with high levels of microbial contamination.1 Biofilm can cause infection among dental patients and exposes oral heath professionals to aerosolized pathogens that may cause illness.2–6
Because water in dental devices may not be of appropriate quality for treatment, the U.S. Centers for Disease Control and Prevention (CDC) developed guidelines for dental unit water quality. The CDC recommends that dental unit waterlines (DUWLs) adhere to the same standards for safe drinking water as set forth by the U.S. Environmental Protection Agency (EPA) of ≤ 500 colony forming units per milliliter (CFU/ml).6 Many advances have been made in scientific understanding of DUWL biofilm and methods for controlling and monitoring water quality in dentistry.
Lacking waterline treatment, biofilm forms in DUWLs relatively quickly. Water from municipal systems (i.e., tap water) contains small amounts of bacteria and other microorganisms. Once the water enters the dental unit, it passes through a complex system of small tubing and some of these organisms collect to form a heterogeneous population on the tubing.3,7,8 Over time, a biofilm matrix forms that provides a nutrient-rich environment conducive to bacterial colonization; this is similar to how biofilm forms plaque on teeth. As water passes over the biofilm, it becomes more heavily infused with bacteria and other microorganisms, and by the time the water exits the handpiece, air/water syringe or ultrasonic scaler, it may contain hundreds of thousands of heterotrophic bacteria in a single milliliter of water — some of which may be human pathogens.7 The use of bottled water, distilled water or sterile water is not effective in preventing biofilm formation in DUWL tubing. In order to maintain acceptable water quality, a comprehensive treatment program must be implemented.
DENTAL TREATMENT WATER INFECTIONS
Little is known about disease outbreaks related to exposure to microorganisms in dental water. This may be due to the low risk associated with bacteria commonly found in untreated lines or because establishing a connection between dental exposure and infection (that arises days after treatment) is difficult. Several incidents in which the connection between infection and exposure to bacteria in dental treatment water have been confirmed, however.
For many years, it was proposed that risk of infection was not significant to individuals with healthy immune systems, as the types of bacteria commonly identified in DUWLs, such as Legionella pneumophila and Pseudomonas aeruginosa, were thought to pose a risk only to immunosuppressed individuals.2,9 Two recent outbreaks, however, have demonstrated that bacteria found in untreated DUWLs may cause illness in patients with healthy immune systems.
In 2015, 20 children were admitted to hospitals in Georgia with infections caused by Mycobacterium abscessus. All affected children had undergone pulpotomies in the same dental clinic. The CDC determined that the source of bacterial contamination was untreated DUWLs with biofilm that had become colonized with M. abscessus.10 The affected children became severely ill and required hospitalization; of these, 17 required surgical excision. In 2016, a cluster of children with infections caused by M. abscessus was reported in California. Although the CDC has not been involved in the California incident, media outlets have reported the infections are connected to contaminated water in a children’s dental clinic.11–13 As many as 58 children developed M. abscessus infections after receiving pulpotomy treatment at the clinic.
The first documented case of infection linked to contaminated dental water was reported in 1987.14 In this case, two patients were diagnosed with postoperative infections. The bacteria isolated from the wound site infections were P. aeruginosa — the same bacteria isolated from the dental unit water supply. The first report of a death associated with dental water contamination occurred in Italy, when an elderly woman was hospitalized and died from Legionnaires’ disease. Because the patient had not been very active in the days leading to her illness, it was possible to determine her exposure occurred during a dental visit.15
With mounting evidence of not only waterline contamination, but also illnesses associated with contaminated dental treatment water, oral health professionals must implement a treatment method with demonstrated effectiveness in controlling DUWL biofilm. In addition to treatment, water quality should be routinely monitored to ensure treatment is effective.16
WATER QUALITY FOR DENTAL PROCEDURES
Since 2003, the CDC has recommended that all dental units use systems that provide output treatment water that meets drinking water standards (≤ 500 CFU/ml of heterotrophic water bacteria) for nonsurgical procedures.17 The CDC further clarified that independent reservoirs — or water-bottle systems — alone are not sufficient and should be combined with commercial products and devices that can improve the quality of water used in routine dental treatment. The CDC recommends consulting the dental unit manufacturer, as well as the manufacturer of the product or device used to control biofilm, for appropriate water maintenance methods and recommendations for monitoring dental water quality (Table 1).6,16
For surgical procedures, the CDC recommends the use of sterile solutions as coolants/irrigants in an appropriate delivery device, such as a sterile bulb syringe, sterile tubing (that bypasses DUWLs), or sterile single-use devices. The CDC’s Guidelines for Infection Control in Dental Health-Care Settings — 2003 define an oral surgical procedure as “involving the incision, excision, or reflection of tissue that exposes the normally sterile areas of the oral cavity. Examples include biopsy, periodontal surgery, apical surgery, implant surgery, and surgical extractions of teeth (e.g., removal of an erupted or nonerupted tooth requiring elevation of mucoperiosteal flap, removal of bone or section of tooth, and suturing, if needed).”6,16
It is important to note the U.S. Food and Drug Administration (FDA) must clear any sterile water/coolant system or device marketed to improve dental water quality.
MANAGING WATER QUALITY
The CDC recommends discharging water and air from dental devices that are connected to the DUWL and enter the patient’s mouth (e.g., handpieces, ultrasonic scalers or air/water syringes) for a minimum of 20 to 30 seconds after each patient.6,17 This is intended to physically flush out patient material (e.g., microorganisms, blood or saliva) that might have entered the turbine, air line or waterline. Flushing alone, however, does not affect biofilm in waterlines or reliably improve the quality of water used during treatment.18,19
In order to achieve the CDC recommended standard of ≤ 500 CFU/ml of water, effective waterline management strategies include the use of commercial devices and procedures, such as:
- Self-contained water systems (e.g., an independent water reservoir), combined with chemical treatment (e.g., periodic or continuous chemical germicide treatment protocols)
- Systems designed for single-chair or entire-practice waterlines that purify or treat incoming water to remove or inactivate microorganisms
Chemical products remove, inactivate or prevent biofilm formation. These treatments are either continuously infused into, or are intermittently added to, the dental unit water. It may be necessary to remove biofilm in DUWLs using a shock treatment before implementing a chemical treatment product. Beyond initial shocking of the waterline, product manufacturers typically provide instructions for routine or periodic shocking of the lines.
Water treatment systems include cartridges that release an active ingredient that disinfects waterlines, and filter devices that remove solid particles from water. The cartridge is connected to the dental unit’s existing water bottle pickup tube. Cartridges need to be replaced at scheduled intervals, according to the manufacturer’s instructions. Filter devices are installed in the waterline between the DUWL and instrument (e.g., dental handpiece). The filter does not affect biofilm in waterlines, but removes microorganisms before water enters the instrument. Filters must be periodically replaced, with the frequency depending on the amount of biofilm in the waterlines and manufacturer’s instructions. These devices may or may not remove endotoxins.
Treatment products and devices for DUWL management are regulated by either the FDA or EPA, while continuous chemical treatment products, systems and filters designed for DUWL treatment are regulated by the FDA. Intermittent chemical treatment products are registered with the EPA as cleaners or disinfectants for DUWL treatment.
The dental unit manufacturer should be consulted to determine the compatible methods, products and devices to maintain water quality in the specific dental unit(s).6,16 For other devices, such as ultrasonic scalers with independent water reservoirs, consult the device manufacturer for instructions and recommended products to control waterline biofilm.
Most new dental units in U.S. practice use a separate water reservoir. This system allows the use of water other than that provided by the municipal water supply. In addition to maintaining better control over the quality of the source water used in patient care, it eliminates interruptions in dental care when local health authorities issue “boil-water” notices. The separate water reservoir — even when using distilled or sterile water — does not, however, prevent biofilm buildup in DUWLs. As noted, in order to achieve recommended water quality for nonsurgical dental procedures, separate water reservoirs must be combined with another approach — such as periodic or continuous chemical treatment, or the use of filters or a centralized water treatment system.6,16
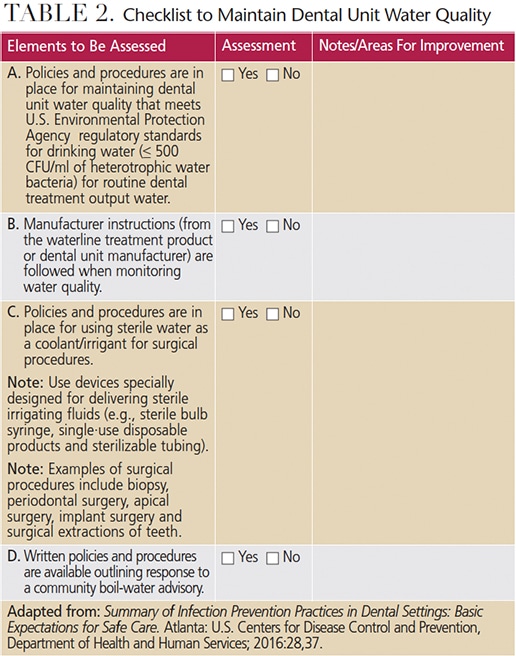
Critical elements of a successful DUWL maintenance regimen include training oral health professionals in site-specific DUWL protocols, and monitoring compliance. Clinicians should follow manufacturer directions and instructions for all commercial products and devices. Staff members responsible for maintaining independent reservoir bottles must handle the bottles with clean gloves, and follow the manufacturer instructions for cleaning and aseptically managing the bottles. The CDC’s 2016 Summary of Infection Prevention Practices in Dental Settings contains a two-part checklist to assess compliance with the CDC 2003 recommendations. Table 2 is adapted from the CDC document, and provides assessment items for site-specific policies and procedures for managing dental water quality, as well as practices that will help ensure compliance.
QUALITY ASSURANCE
Implementing a waterline treatment protocol is an important step in controlling the quality of water used in dental devices. That noted, routine monitoring (through water quality testing) is recommended to ensure effective treatment and that team members are successfully following the protocol. When implementing a water treatment program, the manufacturer instructions should be the first document reviewed. The treatment product directions will determine whether routine monitoring is indicated and, if so, at what frequency.
Several types of waterline testing kits are available. Some involve the collection of water samples that are returned to a laboratory for analysis. Direct-read tests provide results after the collection of water sample in the dental office. The American Dental Association provides a list of testing devices and services, but does not validate the accuracy of such methods, as not all testing methods are reliable and accurate.20,21 Oral health professionals should consult dental treatment product manufacturers regarding recommended test methods and frequency of use.
A lack of awareness regarding the CDC’s recommendations for dental unit water quality — including monitoring programs — was reported in a survey conducted by the CDC’s Division of Oral Health. Seeking to identify factors associated with implementation of the first four practices recommended in the CDC’s Guidelines for Infection Control in Dental Health-Care Settings — 2003, it surveyed a stratified random sampling of 6825 U.S. dentists. Only one dentist per practice was chosen for the survey, and the response rate was 49%.22
One of the CDC-recommended infection control practices included in the survey was whether a single water system was implemented for each dental unit, and if water quality had been evaluated at least once over the past year.22 While the CDC recommends that dental practices monitor water quality, it does not provide guidelines regarding the frequency of monitoring.16 It does, however, suggest that practices work with the dental unit/water delivery system manufacturer to ascertain the most effective strategy for ensuring acceptable water quality.22 Of the practices that participated in the survey, 33% utilized separate water systems and monitored water quality at least once per year. On the other hand, 32% reported they did not use separate water systems or monitor water quality.
These results highlight the need to increase awareness of CDC recommendations for controlling dental unit water quality, and the importance of implementing protocols for ensuring quality water for nonsurgical and surgical procedures. Management programs for DUWLs should follow manufacturer instructions for all products and equipment. Evaluating effective and consistent compliance —and periodically monitoring the quality of the dental unit water — are important components of an effective maintenance strategy.
Clinicians, professional organizations, dental educators, and manufacturers/distributors of products and devices for managing dental unit water quality play key roles in facilitating awareness of the importance of clean DUWLs, and promoting compliance with CDC recommendations for dental unit water quality. Simply stated, failing to ensure the safety of dental treatment water puts patients and practitioners at risk.
References
- Blake GC. The incidence and control of bacterial infection in dental spray reservoirs. Brit Dent J. 1963;115:413–416.
- Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol. 1995;61:1208–1213.
- Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66:3363–3367.
- Challacombe SJ, Fernandes LL. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J Am Dent Assoc. 1995;126:603–608.
- Coleman DC, O’Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit waster systems and its practical control. J App Microbiol. 2009;106:1424–1437.
- U.S. Centers for Disease Control and Prevention. Dental Unit Water Quality. Available at: cdc.gov/oralhealth/ infectioncontrol/questions/dental-unit-water-quality.html. Accessed March 13, 2017.
- Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. J Am Dent Assoc. 2000;131:1427–1441.
- Lal S, Pearce M, Achilles-Day UEM, et al. Developing an ecologically relevant heterogeneous biofilm model for dental-unit waterlines. Biofouling. 2017;33:75–87.
- Lenz AP, Williamson KS, Pitts B, Stewart PS, Franklin MJ. Localized gene expression in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2008;74:4463–4471.
- Peralta G, Tobin-D’Angelo M, Parham A, et al. Mycobacterium abscessus infections among patients of a pediatric dentistry practice — Georgia, 2015. MMWR Recomm Rep. 2016;65:355–356.
- Ross E. Infection outbreak shines light on water risks at dentists offices. Available at: npr.org/sections/health-shots/ 2016/09/30/495802487/infection-outbreak-shines-light-on-water-risks-at-dentists-offices. Accessed March 13, 2017.
- Kravarik J. Bacteria in dentist’s water sends 30 kids to hospital. CNN. Available at: cnn.com/2016/10/11/health/california-dental-water-bacteria. Accessed March 13, 2017.
- Rocha V, Lozano C. Orange County children’s dental clinic closed after bacteria found in new water system. Los Angeles Times. December 17, 2016.
- Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J. 1987;163:152–154.
- Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet. 2012;379:684.
- Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Atlanta: U.S. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2016.
- U.S. Centers for Disease Control and Prevention. Recommended infection-control practices for dentistry, 1993. MMWR Recomm Rep. 1993;42:1–12.
- Williams JF, Johnston AM, Johnson B, Huntington MK, Mackenzie CD. Microbial contamination of dental unit waterlines: prevalence, intensity and microbiological characteristics. J Am Dent Assoc. 1993;124:59–65.
- Williams HN, Johnson A, Kelley JI, et al. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int. 1995;26:331–337.
- Porteous N, Sun Y, Schoolfield J. Evaluation of 3 dental unit waterline contamination testing methods. Gen Dent. 2015;63:41–47.
- Momeni SS, Tomline N, Ruby JD, Dasanayake AP. Evaluation of in-office dental waterline testing. Gen Dent. 2012;60:142–147.
- Cleveland J, Foster M, Barker L, et al. Advancing infection control in dental care settings: Factors associated with dentists’ implementation of guidelines from the Centers for Disease Control and Prevention. J Am Dent Assoc. 2012;143:1127–1138.
Featured photo by edwardolive / istock / Getty Images Plus
From Decisions in Dentistry. April 2017;3(4):32–35.




![The controversy around adding fluoride to drinking water was recently highlighted by an article in the Wall Street Journal, which addressed its possible negative neurological effects on children.[1] The article cited recent studies on what levels of fluoride are needed to protect teeth without risking possible cognitive harm to prenatal children and infants. Link in bio.
[1] Data on link.
---
#dentistry #dentist #dental #smile #dentista #teeth #cosmeticdentistry #dentistryworld #dentalphotography #odonto #tooth #dentistrylife #orthodontics #dentalcare #dentalhygienist #dentalimplants #oralhealth #veneers #dentalstudent #dentalassistant #dentalclinic #dentistlife #dentalhygiene #teethwhitening #oralsurgery #dds #endodontics #continuingeducation #education](http://decisionsindentistry.com/wp-content/plugins/instagram-feed/img/placeholder.png)
