Trends in Local Anesthesia Delivery
Innovations in technology and materials are helping clinicians better manage procedural and postprocedural pain.
With a remarkable record of safe use, the main mechanism oral health professionals have for addressing the pain associated with dental care is local anesthesia.1 Considered by some as the most unpleasant event during dental encounters, the anesthetic injection is an important factor in how patients judge dental providers.2,3 Considering the importance of pain management to patients, and changes in care brought about by the opioid crisis, clinicians should keep up with trends in care and adapt their practices accordingly. This paper will present an overview of trends and advances in the delivery of local anesthetics.
The ability to accurately control the length of anesthetic effect is often listed as a desirable anesthetic characteristic. Although not yet attainable, the desire for more control has led to the use of phentolamine mesylate (PM) as a “reversal agent” for local anesthetics. This is an alpha adrenergic blocker that impedes adrenergic receptors working within the circulatory system. This usually results in a reduction of total peripheral resistance and lower venous return to the heart.4 In dentistry, PM is used to reduce soft tissue anesthesia duration following the administration of local anesthetics.5,6 It increases elimination of local anesthetic via localized reduction of vascular resistance. Pivotal studies have shown a reduction in the overall time of soft tissue anesthesia following administration of PM.5–7 In addition, a recent meta-analysis of six randomized clinical trials found it effective in decreasing anesthesia time with mandibular procedures that involved anesthetizing the lip and tongue. Importantly, the differences in reported adverse events between PM and placebo were not statistically significant.8
The PM formulation currently utilized in dentistry is administered in 0.4-mg cartridges at a 1:1 ratio of the agent to local anesthetic cartridge at the injection site. The recommended maximum dose is two cartridges, and the agent is not recommended in patients younger than 3 or who weigh less than 33 pounds. In pediatric patients weighing between 33 and 66 pounds, the maximum recommended dose is one-half cartridge or 0.2 mg. It should be noted that a dosage of more than one cartridge has not been studied in children younger than 4 years. The most frequently reported adverse events include headache, pain during injection, and postinjection pain.8,9
Recent advances have made it easier to alter the acidity of anesthetic formulations. Alkalinization can reduce injection pain by increasing the pH of the anesthetic solution prior to deposition. As seen in Table 1, dental anesthetics with lower pH values are more likely to be associated with higher pain values.10–12 In addition to reducing injection pain, it has been suggested that alkalinization can accelerate the onset of analgesia.13 Two possible mechanisms that may improve onset times with buffered anesthetic are a potentiated effect of carbon dioxide produced with alkalinization using sodium bicarbonate, and improved proportions of active anesthetic molecules. Carbon dioxide has been reported to have an independent anesthetic effect on peripheral nerves and may potentiate the effects of lidocaine.14–16 The pH of the tissue and solution affects the amount of active anesthetic molecules present, which are vital to permeate membranes and produce an anesthetic effect.17 Alkalinization can increase the pH and may allow for a higher amount of active molecules.13 It is recommended that dental teams complete the buffering process for each injection immediately before administration.13 At this stage, additional research is needed to more thoroughly understand alkalinization’s role and effect on daily practice.
ROUTE OF ADMINISTRATION
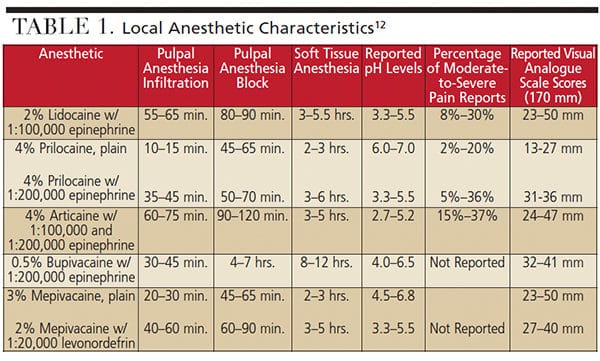
Dependable local anesthetic administration that does not involve needle delivery serves as a valuable option to patients. Recently, an intranasal anesthetic agent, 3% tetracaine with 0.5% oxymetazoline in a preparation with mucoadhesive properties, is being utilized for some dental procedures. Research demonstrates that delivery via spray into the ipsilateral nostril of the treatment area achieved adequate anesthesia 84% to 90% of the time for a single restorative procedure.18,19 Once sprayed within the nostril, the anesthetic solution utilizes small communications within the nasal anatomy to spread inferiorly. As seen in Figure 1, the anterior and middle superior alveolar nerves are affected, providing pulpal anesthesia from the premolars to incisors. It should be noted that patients may not possess a middle superior alveolar innervation, and the posterior alveolar nerve would innervate the second premolar, possibly making the intranasal administration ineffective. The U.S. Food and Drug Administration has approved this intranasal agent for use in individuals weighing 88 pounds or more, with a maximum recommended dose of 18 mg tetracaine with 0.3 mg oxymetazoline. The most commonly reported adverse occurrences are nasal congestion and rhinitis.20
The administration of intrapocket anesthetic (IPA) has been used most often by dental hygienists for scaling and root planing, and by dentists for placement of gingival retraction cord. Administration involves the application of topical anesthesia within the gingival pocket of the tooth, but IPA does not produce profound pulpal anesthesia. For most formulations, it can have an onset time as early as 30 seconds and duration of action of approximately 20 minutes.21,22 While IPA provides some anesthetic benefit compared to placebo, using an injectable local agent for scaling and root planing was found to provide superior anesthesia in a recent meta-analysis.23
Introduced to dentistry in the early 1900s, the intraosseous injection did not achieve widespread acceptance due to its invasive nature. This injection accesses the nerve of a tooth via the cortical bone, with the traditional methodology employing a drill to form an opening into the bone between adjacent root structures. More recent techniques utilize devices that assist with bone and tissue penetration, either by providing instruments and mechanics that allow for less traumatic bone perforation, or via needles with a scalpel bevel (Figure 2) that more easily overcome the resistance encountered.24,25

Also considered an intraosseous injection, the intraligamentary injection (ILI), sometimes referred to as a periodontal ligament injection, utilizes the periodontal ligament space to assist in anesthetic spread via soft tissue and communications within the bone of the tooth socket. This environment allows anesthesia to spread to the cancellous alveolar bone and access the nerve fibers of the tooth (Figure 3). According to Bassett et al,26 ILIs are “perhaps the most universal of the supplemental injections,” and commonly used as a supplemental injection when adequate anesthesia is not achieved with an inferior alveolar nerve block. It is also utilized for single tooth anesthesia when low anesthetic dosage is required and/or a large area of anesthetic effect is not recommended.26
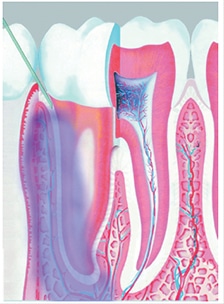
While there are many variations with ILI methodology, some common steps can be observed. Recommendations provide the needle be inserted at a 30° angle along the long axis of the mesial or distal root for single-rooted teeth, and on the mesial and distal roots of multirooted teeth. Once the needle is placed between the root and crestal bone, significant resistance is experienced. Anesthetic deposition is recommended at 0.2 ml of the anesthetic cartridge, per root or site, injected for over a period of at least 20 seconds.1 Success rates of approximately 90% have been demonstrated when an ILI is used as a supplemental injection after a failed mandibular block.27,28 Successful administration requires the anesthetic to be deposited under pressure; in addition, the solution should not flow out of the sulcus into the mouth. Due to the pressure necessary to deposit the solution in the ligament area, some practitioners find computer-controlled local anesthetic delivery (CCLAD) useful to enhance injection control and assist in decreasing the pain of injection.26
TECHNOLOGY-ASSISTED DELIVERY
Since its introduction, CCLAD technology has continued to attract interest from dental teams. The device utilizes computer microprocessors to manage fluid dynamics and anesthetic deposition. By reducing subjective flow rates, technology-assisted delivery decreases the variability in pressure that occurs with manual administration. This slow and controlled administration reportedly minimizes the pain experience for patients. Marginal success rates have been demonstrated using CCLADs for some techniques, such as the anterior middle superior alveolar injection.29 New-generation units utilize dynamic pressure-sensing mechanisms to optimize needle placement and accuracy of fluid deposition.30 These systems offer providers real-time feedback relating to force and pressure during anesthetic flow.31
Training is necessary when incorporating CCLADs into practice, and a learning curve during patient care should be expected. With some devices, initial difficulties have been reported with the operation of a foot rheostat to trigger the syringe and administer anesthetic, although clinical experience will improve administration skills.32 While patient reports of pain on injection decrease, these devices do not completely eliminate injection pain.
NONANESTHETIC AGENTS
Although limited, the utilization of nonanesthetic injectable agents has been reported in dental practice. As anti-inflammatory agents, the majority of these solutions inhibit prostaglandin synthesis,33–35 and their use intraorally, at the site of care, is being explored to improve oral pain management. In an analysis of postendodontic pain perception with irreversible pulpitis and use of intraligamentary injections, subjects who received 0.4 ml of 20 mg ml-1 of piroxicam (a nonselective, nonsteroidal anti-inflammatory drug) reported a lower intensity of pain (on the visual analogue scale) compared to a group that received 0.4 ml of a 2% lidocaine with 1:80,000 epinephrine.36
Mandibular infiltration injection of ketorolac (a nonselective, nonsteroidal anti-inflammatory drug) resulted in more pain relief than intramuscular injection of ketorolac.33 An analysis that evaluated injection of methylprednisolone (a corticosteroid used to decrease inflammation) at the site of care reported a “significant reduction in frequency and intensity of postoperative pain” after a root canal procedure.37 Early research and use demonstrate the promise of nonanesthetic agents for postoperative pain management — although additional research into appropriate use and best practices is warranted.
ANESTHETIC MEDIUM
The long-acting local anesthetic 0.5% bupivacaine with 1:200,000 epinephrine is available in dental cartridges and is often used by oral surgeons following third molar extractions. It has been found to effectively manage postoperative pain following oral surgery and minimize the need for systemic opioids, such as codeine, oxycodone and hydrocone.38 The opioid crisis was created in part by the use and misuse of opioid analgesics prescribed for postoperative pain. If local anesthesia could be safely and reversibly extended a day or two, writing prescriptions for analgesic medications, either opioids or nonopioids, conceivably might no longer be necessary.
The strategy of prolonging the numbing effect of local anesthesia is an important component of multimodal therapies now being advocated to limit the need for opioid analgesics. As indicated in Table 1, the duration of soft tissue anesthesia provided by 0.5% bupivacaine is nearly twice that of other local anesthetics available in dentistry. Because prolonging anesthesia will delay the development of acute postoperative pain and possibly minimize its severity for several hours, the need for opioids is decreased. Subsequently, the use of highly effective nonsteroidal anti-inflammatory drugs as the first-line of acute pain therapy is a justifiable strategy for patients experiencing moderate-to-severe pain following dental surgery.39
An innovative approach for prolonging bupivacaine-induced anesthesia has been developed by formulating the local anesthetic into slowly resorbable liposomes that, when injected, may provide a tissue storage depot for the anesthetic agent. The current liposomal bupivacaine formulation is available in 10-ml vials and prepared as a 1.3% concentration.40 This preparation embeds bupivacaine into small multivesicular liposomes that reportedly provide a sustained release of the anesthetic.41
Although there are inconsistencies in the results, liposomal bupivacaine has been shown to reduce postoperative pain following general surgical procedures, such as knee arthroscopy, hemorrhoidectomy, breast augmentation and bunionectomy.42 While published reports generally support findings that the severity of acute pain decreases (which would reduce the need for opioid analgesics), results supporting opioid-sparing outcomes have been less consistent in acute dental pain studies.43,44 The largest study of liposomal bupivacaine in dentistry evaluated analgesic properties following third molar extractions. Although the results indicated significant improvement in pain scores following third molar extractions, the authors noted that, due to extensive protocol violations, additional studies are needed to demonstrate effectiveness.45
A recent Cochrane meta-analysis concluded that while liposomal bupivacaine administered at the surgical site appears to reduce postoperative pain,46 at present, the limited evidence does not convincingly demonstrate superiority to bupivacaine hydrochloride. Given the significant cost, questions remain regarding the utility of liposomal bupivacaine in dentistry. Alternative formulations, and further experience regarding injection site and volumes, may be necessary to advance this innovative concept into routine postoperative pain management.
CONCLUSION
The desire to provide a painless dental visit is shared by patients and practitioners alike. Agents that allow better control of anesthetic onset time and duration of action are available to dental care teams, as are needle designs and technologies that impact anesthetic delivery. The utilization of nonanesthetic agents to improve pain response during care, along with new anesthetic mediums — such as mucoadhesive preparations and multivesicular liposomes — appear to be interesting advances in dentistry. The profession continues to see innovations in technology and materials to improve the patient’s experience and maintain a long history of safety in oral pain management.
KEY TAKEAWAYS
- Phentolamine mesylate can be used to reduce soft tissue anesthesia duration following the administration of local anesthetics.5,6
- Alkalinization (or buffering) can reduce injection pain by increasing the pH of the anesthetic solution prior to deposition.
- An intranasal anesthetic agent, 3% tetracaine with 0.5% oxymetazoline in a preparation with mucoadhesive properties, is now being utilized for some dental procedures.
- By reducing subjective flow rates, computer-controlled local anesthetic delivery decreases the variability in pressure that occurs with manual administration.
- The intraoral use of anti-inflammatory, nonanesthetic injectable agents at the site of care is being explored to improve oral pain management.
- Following oral surgeries, prolonging the numbing effect of local anesthesia is an important component of multimodal therapies now being advocated to reduce the need for opioid analgesics.
- In an innovative approach, liposomal bupivacaine has been shown to reduce postoperative pain after general surgical pro-cedures. Further study is needed regarding its use in dentistry.
REFERENCES
- Malamed SF. Handbook of Local Anesthesia e-book. Elsevier Health Sciences; 2014 Apr 25.
- de St Georges J. How dentists are judged by patients. Dent Today. 2004;23:96–99.
- Boynes SG. Intraligamentary Injections. Available at: https://www.dentalacademyofce.com/. Accessed October 23, 2018.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Hersh EV, Lindemeyer RG. Phentolamine mesylate for accelerating recovery from lip and tongue anesthesia. Dent Clin N Am. 2010;54:631642.
- Hersh EV, Moore PA, Papas AS, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate in adolescents and adults. J Am Dent Assoc. 2008;139:1080–1093.
- Tavares M, Goodson JM, Studen-Pavlovich D, et al. Reversal of soft-tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.
- Prados-Frutos JC, Rojo R, González-Serrano J, et al. Phentolamine mesylate to reverse oral soft-tissue local anesthesia: a systematic review and meta-analysis. J Am Dent Assoc. 2015;146:751–759.
- Hersh EV, Lindemeyer R, Berg JH, et al. Phase four, randomized, double-blinded, controlled trial of phentolamine mesylate in two- to five-year-old dental patients. Pediatr Dent. 2017;39:39–45.
- Whitcomb M, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1:100,000 epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2010;57:59–66.
- Goodchild JH, Donaldson M. Comparing the pH change of local anesthetic solutions using two chairside buffering techniques. Compendium. 2016;37:e6.
- Boynes SG. Analysis of the two-stage injection technique. Decisions in Dentistry. 2016;2(12):26–29.
- Malamed SF, Falkel M. Advances in local anesthetics: pH buffering and dissolved CO2. Dent Today. 2012;31:88–93.
- Bokesch PM, Raymond SA, Strichartz GR. Dependence of lidocaine potency on pH and PCO2. Anesth Analg. 1987;66:9–17.
- Raymond S, Wong K, Strichartz G. Mechanisms for potentiation of local anesthetic action by CO2: bicarbonate solutions. Anesthesiology. 1989;71(Suppl):A711.
- Condouris GA, Shakalis A. Potentiation of the nerve depressant effect of local anaesthetics by carbon dioxide. Nature. 1964;204:57–58.
- Rood JP. The use of buffered lignocaine solution in the presence of acute inflammation. J Dent. 1977;5:128–130.
- Saraghi M, Hersh EV. Intranasal tetracaine and oxymetazoline spray for maxillary local anesthesia without injections. Gen Dent. 2017;65:16–19.
- Hersh EV, Pinto A, Saraghi M, et al. Double-masked, randomized, placebo-controlled study to evaluate the efficacy and tolerability of intranasal K305 (3% tetracaine plus 0.05% oxymetazoline) in anesthetizing maxillary teeth. J Am Dent Assoc. 2016;147:278–287.
- Hersh EV, Saraghi M, Moore PA. Two recent advances in local anesthesia: intranasal tetracaine/oxymetazoline and liposomal bupivacaine. Curr Oral Health Rep. 2017;4:189–196.
- Friskopp J, Huledal G. Plasma levels of lidocaine and prilocaine after application of Oraqix, a new intrapocket anesthetic, in patients with advanced periodontitis. J Clin Periodontol. 2001;28:425–429.
- Chintala K, Kumar SP, Murthy KR. Comparative evaluation of effectiveness of intra-pocket anesthetic gel and injected local anesthesia during scaling and root planing — a split-mouth clinical trial. Indian J Dent Res. 2017;28:281–287.
- Wambier LM, de Geus JL, Boing TF, et al. Intrapocket topical anesthetic versus injected anesthetic for pain control during scaling and root planing in adult patients: systematic review and meta-analysis. J Am Dent Assoc. 2017;148:814–824.
- Nusstein JM. Local Anesthesia. In: Peters OA, ed. The Guidebook to Molar Endodontics. Berlin, Heidelberg: Springer; 2017:75–91.
- Dentistry Today. Septodont and Tuttle to Develop Needle Bending Guide. Available at: http://www.dentistrytoday.com/news/industrynews/item/2997-septodont-and-tuttle-to-develop-needle-bending-guide. Accessed October 23, 2018.
- Bassett K, DiMarco A, Naughton D. Local Anesthesia for Dental Professionals. 2nd ed. Pearson; 2015.
- Walton RE, Abbott BJ. Periodontal ligament injection: a clinical evaluation. J Am Dent Assoc. 1981;103:571–575.
- Smith GN, Walton RE, Abbott BJ. Clinical evaluation of periodontal ligament anesthesia using a pressure syringe. J Am Dent Assoc. 1983;107:953–956.
- Lee S, Reader A, Nusstein J, Beck M, Weaver J. Anesthetic efficacy of the anterior middle superior alveolar (AMSA) injection. Anesth Prog. 2004;51:80–89.
- Hochman MN. Single-tooth anesthesia: pressure-sensing technology provides innovative advancement in the field of dental local anesthesia. Compend Contin Educ Dent. 2007;28:186–193.
- Bassett K, DiMarco A. Maximize the benefits of computer-controlled local anesthesia delivery. Dimensions of Dental Hygiene. 2016;14(07):37–38.
- Kammerer PW, Schiegnitz E, von Haussen T, et al. Clinical efficacy of a computerized device (STA) and a pressure syringe (VarioJect INTRA) for intraligamentary anaesthesia. Eur J Dent Educ. 2015;19:16–22.
- Penniston SG, Hargreaves KM. Evaluation of periapical injection of ketorolac for management of endodontic pain. J Endod. 1996;22:55–59.
- Torabinejad M, Bakland LK. Prostaglandins: their possible role in the pathogenesis of pulpal and periapical diseases, part 2. J Endod. 1980;6:769–771.
- Cohen J, Reader A, Fertel R, Beck F, Meyers W. A radioimmunoassay determination of the concentrations of prostaglandins E2 and F2 in painful and asymptomatic human dental pulps. J Endod. 1985;11:330–335.
- Atbaei A, Mortazavi N. Prophylactic intraligamentary injection of piroxicam (feldene) for the management of post-endodontic pain in molar teeth with irreversible pulpitis. Aust Endod J. 2012;38:31–35.
- Kaufman E, Heling I, Rotstein I, et al. Intraligamentary injection of slow-release methylprednisolone for the prevention of pain after endodontic treatment. Oral Surg Oral Med Oral Pathol. 1994;77:651–654.
- Moore PA, Nahouraii HS, Zovko J, Wisniewski SR. Dental therapeutic practice patterns in the U.S. I. Anesthesia and sedation. Gen Dent. 2006;54:92–98.
- Moore PA and Hersh EV. Combining ibuprofen and acetaminophen for acute postoperative pain management: translating clinical research to dental practice. J Am Dent Assoc. 2013;144:898–908.
- Exparel Package Insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022496s005lbl.pdf. Acessed October 23, 2018.
- Davidson EM, Barenholz Y, Cohen R, Haroutiunian S, Kagan L, Ginosar Y. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth Analg. 2010;110:1018–1023.
- Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107–116.
- Glenn B, Drum M, Reader A, Fowler S, Nusstein J, Beck M. Does liposomal bupivacaine (Exparel) significantly reduce postoperative pain/numbness in symptomatic teeth with a diagnosis of necrosis? A prospective, randomized, double-blind trial. J Endod. 2016;42:1301–1306.
- Bultema K, Fowler S, Drum M, Reader A, Nusstein J, Beck M. Pain reduction in untreated symptomatic irreversible pulpitis using liposomal bupivacaine (Exparel): a prospective, randomized, double-blind trial. J Endod. 2016;42:1707–1712.
- Lieblich SE, Danesi H. Liposomal bupivacaine use in third molar impaction surgery: INNOVATE study. Anesth Prog. 2017;64:127–135.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;2:CD011419.
Related to the topics of this article, Sean G. Boynes, DMD, MS, has provided continuing education lectures and interactive publications sponsored by St. Renatus, Cetylite Industries, Wyeth Pharmaceutical, Septodont, Henry Schein and Milestone Scientific.
During his career, Paul A. Moore, DMD, PhD, MPH, has completed sponsored research for many of the innovations discussed in this review. Currently, he reports no financial or commercial conflicts of interest with any product manufacturer.
Featured image by YURI10B/ISTOCK/GETTY IMAGES PLUS
From Decisions in Dentistry. December 2018;4(12):18—21.