Oral Health Considerations, Risks, and Signs of Alzheimer’s Disease
By understanding the risk factors and brain changes associated with this disease, clinicians will be better prepared to provide effective oral healthcare.
This course was published in the March 2023 issue and expires March 2026. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 750
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the prevalence, risks, and signs of Alzheimer’s disease (AD), as well as oral health considerations for affected patients.
- Discuss the connection between periodontal infection and the brain infection seen in patients with AD.
- Explain the lost and retained abilities related to brain changes among individuals with AD.
This is the first article in a short series exploring the risks and signs of Alzheimer’s disease, as well as oral healthcare considerations. Appearing in a future issue, the concluding installment will discuss oral care assistance for this population, and suggest necessary modifications to dental appointments.
Dental professionals routinely provide care for individuals with dementia. Characterized by abnormal brain changes severe enough to interfere with daily life, dementia often causes memory loss, inability to use language, and challenges with problem-solving.1 Alzheimer’s disease (AD) is the most common type of dementia, accounting for 60% to 80% of all types.1 In 2019, dementia affected 9.8% of Americans age 70 and older.2 In 2022, approximately 6.5 million Americans age 65 and older were living with AD.3
Changes in the brain are responsible for AD. Amyloid beta-peptide plaques form in neuron synapses, interfering with impulse transmission.4,5 Within the neurons, tangles of tau protein develop.4 Accumulation of plaques between neurons coupled with the tau found inside the neurons interfere with the neurons’ ability to communicate. As a result, the neurons degenerate and the brain shrinks.6 This affects the individual’s ability to think, remember, make decisions, and function independently.6 Plaques and tangles form through inflammation and exposure to inflammatory mediators, such as TNF-α, IL-1β and IL-6.7 These accompany any inflammatory disease, including periodontitis. Thus, inadequate oral biofilm removal is the precursor to this process. As such, patients should be educated about the importance of oral health and need to remove potentially harmful oral biofilm.
UNDERSTAND THE RISK FACTORS
Several factors contribute to the risk of developing AD, including advanced age, genetics and environmental exposures.8 The gene with the strongest impact on AD risk is APOE-e4. Between 40% and 65% of people with AD have the APOE-e4 gene.8 Environmental exposures may include air pollution, pesticides and vitamin D deficiency; however, these require further research to validate their connections to AD. Previous damage to the brain (e.g., traumatic brain injuries) also increases AD risk.8 In addition, risk increases when the blood vessels that supply the brain are damaged, such as occurs with hypertension, stroke, uncontrolled diabetes mellitus and high cholesterol.8
Level of education poses a risk for AD. Individuals who have completed more years of formal education are at reduced risk of AD than those with fewer years of education.4 This connection is not well understood, but may be linked to the development of neuronal connections in the brain from continued education. Inadequate sleep or poor sleep quality, excessive alcohol use, depression, and hearing impairment are also possible risk factors currently under investigation.4
Periodontitis and infection with the Gram-negative bacteria Porphyromonas gingivalis were identified as risk factors for AD as far back as 2006.5,9 The National Institute of Dental and Craniofacial Research states that 42% of adults age 30 and older have periodontitis.10 This puts a large percentage of the population at risk for AD. P. gingivalis can be found in the brain decades before cognitive decline is observed.9 Furthermore, AD-related brain changes are thought to begin 20 years or more before symptoms present.4 A 2017 study of 9291 individuals with periodontitis determined the presence of periodontitis for 10 years or more led to a 70% higher risk of developing AD compared to those without periodontitis.7 This finding emphasizes the importance of stabilizing periodontal disease in adults, especially those at increased risk for AD. The invasion of brain tissue by P. gingivalis is not a result of poor oral care after AD is diagnosed;9 rather, poor oral care is the precursor to, and sustainer of, periodontitis.
P. gingivalis produces a virulence factor known as gingipains. When secreted, gingipains play a critical role in host colonization, inactivate the host’s inflammatory response, and destroy tissues.9 P. gingivalis travels from the mouth to the brain through a variety of pathways. The organism may enter the bloodstream during a bacteremia event, which can be caused by toothbrushing, flossing, chewing or dental procedures.9,11 During a bacteremia, the organism travels to other bodily systems, including the coronary arteries, placenta, liver, brain and cerebrospinal fluid.9 P. gingivalis can also infect monocytes that are recruited to the brain, directly infecting and damaging endothelial cells that protect the blood-brain barrier and spreading through cranial nerves. After entry into the brain, the spread of the organism from neuron to neuron is slow. The presence of P. gingivalis in the brain increases the production of amyloid beta, a component of the plaques that contribute to AD. Both P. gingivalis and gingipains are found in the brain tissue of those with AD; P. gingivalis is also found in the cerebrospinal fluid of individuals with AD.9
Salivary testing has been designed to detect the oral microorganisms capable of causing periodontal disease, as well as other systemic conditions. Consequently, salivary testing may be an excellent pathway to early identification for those with AD risk factors.
SIGNS OF ALZHEIMER’S DISEASE
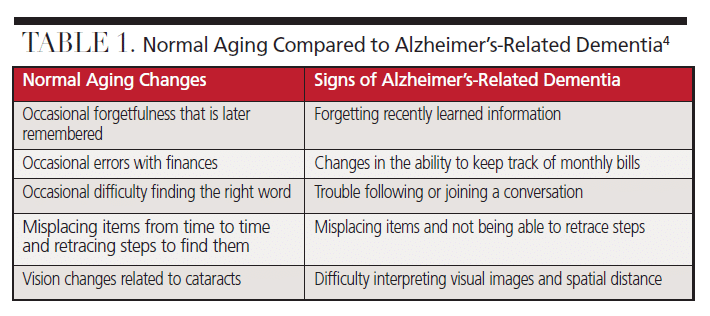
Aging brings cognitive changes that may be unrelated to AD. Table 1 compares normal aging changes to signs of Alzheimer’s-related dementia.4 For those experiencing normal aging changes, it may be a relief to find these cognitive changes are typical of the aging process. It is also important for dental professionals to know the difference between normal aging and the signs of AD.
There are four truths about dementia that differ from normal aging: 1) at least two parts of the brain are actively dying; 2) the structure of the brain is changing; 3) the condition is chronic; and 4) dementia is terminal.12 During this process, signs of AD will surface, including changes in memory, language, vision and sensory perception. Eventually, individuals with AD lose the ability to care for themselves as daily tasks — such as initiating and fulfilling a skill, tool manipulation, and sequencing — become highly challenging.13
Those with AD lose memory and details of recent events, while long-ago memories, emotional memories, and motor memories are retained. This explains why individuals living with AD like to retell stories from their past and can complete tasks using motor skills. Language is greatly affected due to the shrinking of the brain. Individuals living with AD find it difficult to retrieve the right word during conversations. They may accidentally mix up words or add words to their sentences. Language becomes vague instead of descriptive and is limited to short, simple phrases. Preserved language skills include singing, use of forbidden words, and automatic responses, such as greetings. Individuals living with AD struggle to understand verbal language, but may retain the ability to interpret facial expressions, tone of voice, and other nonverbal cues.13 This emphasizes the need for simple language and awareness of body language when communicating with those with AD.
While memory changes are commonly associated with AD, other changes may be overlooked or dismissed. Those with AD lose peripheral vision, leading to sight only in middle of the field of vision. Object recognition and purpose become less familiar,13 which may affect the ability to perform tasks, such as rinsing after toothbrushing. If an individual with AD thinks the cup contains a beverage instead of mouthrinse, the individual is likely to swallow the liquid instead of rinsing with it. This creates a need for direct supervision. Depth perception worsens as vision loss accelerates. As AD progresses, the field of vision narrows, leading to a small amount of vision in only one eye at a time. This is called monocular vision.
Hearing is not affected by dementia-related brain changes. Sensory changes include the loss of body and position awareness, as well as the ability to locate and express pain. Sensation remains active and even heightened in some areas of the body, including the mouth, lips, tongue, hands, feet and genitalia.13 These heightened areas can elicit either a positive or negative response when touched. Oral health professionals should be prepared for exaggerated reactions when touching the mouth, lips and tongue of a patient with AD.
Individuals with dementia may experience changes in personality and mood. Depression is the most common mood disorder among this population.14 The onset of depression can be a warning sign of AD. If other signs of AD are present, an evaluation by a physician is prudent. A positive association between depression and caries also exists.15 The exact etiology is not well understood, but caries preventive strategies can be implemented if depression is diagnosed. Anxiety can be present early in the course of AD and is more commonly identified in those diagnosed before the age of 65.14
DISEASE STAGES
Early onset of AD begins before the age of 65 and late onset occurs at age 65 and older.4 Early onset AD is thought to have strong genetic connections, while late onset AD is influenced by environmental factors.5 The first stage of AD is preclinical where measurable biomarkers indicate the earliest signs of AD, but symptoms have not yet developed. Biomarkers of AD are found in the cerebrospinal fluid and seen on positron emission tomography (PET) scans during the preclinical stage. Mild cognitive impairment is the next stage and is characterized by subtle symptoms, such as problems with memory, language and thinking. About one-third of those with mild cognitive impairment develop AD within five years.4
Once dementia is established, noticeable changes occur that impair an individual’s ability to function in daily life.4 Those with mild dementia can function in many areas, but are likely to require assistance with some activities to maximize independence and safety. As the longest stage of AD, moderate dementia causes more problems with memory and language, increases feelings of confusion, and hinders individuals’ abilities to complete multistep tasks, such as bathing and dressing. The final stage of dementia greatly diminishes the ability to communicate verbally. Individuals with severe dementia may require around-the-clock care and likely are not visiting the dental office for care unless it is pain related.
GEMS CLASSIFICATION SYSTEM
The shift in skills and cognitive abilities can be categorized with the GEMS brain change model developed by Snow.16 One of the leading educators on dementia and its care in the United States and Canada, Snow is founder of the Positive Approach to Care, whose mission is to enhance life and the relationships of those living with brain change by fostering an inclusive global community. Each stage of dementia has unique changes that can be grouped into six gemstones within the GEMS brain change model.16 These gemstones correlate to the stages of AD. A clear understanding of these changes might align dental professionals with the appropriate expectations regarding the ability and cooperation of patients with dementia.
The first GEM is the sapphire. At this stage, the brain is healthy and cognition is optimal. Normal aging is evident in those who are in the sapphire stage. The second, or diamond, stage reflects clear and sharp cognition when happy, but behavior can be cutting when distressed. Diamonds prefer the familiar and may resist change. These individuals need repetition and time to absorb new information. Those in the emerald stage display flaws, such as missing one of every four words in a conversation, lacking safety awareness, and experiencing irrational thoughts. Strong emotional reactions may occur in the emerald stage when the individual feels fearful or has unmet needs. Changes in the amber stage include increased sensitivity in areas such as the mouth, hands and feet. Intolerance to discomfort in these areas may lead to resistance in activities involving the mouth, such as eating, taking medication and/or oral hygiene. Ambers may refuse care or see someone who is trying to help them as threatening. Individuals in the ruby stage have lost fine motor skills and need someone to guide their movements and transitions. They need assistance starting and stopping skills. The pearl stage is near the end of life. The brain is losing the ability to control and heal the body. Difficulty breathing and swallowing is common.16 (More information about the GEMS brain change model is available at teepasnow.com.) Sharing this information with family members and caregivers may help them better understand the state of the disease and provide optimal support for the person with AD.
DIAGNOSTIC PROCEDURES AND TREATMENT
Primary care physicians usually diagnose AD, while related specialists may include neurologists, geriatric psychiatrists, geriatricians and neuropsychologists. In addition to reviewing the patient’s medical history, physicians use questionnaires, clinical exams and brief assessments to evaluate thinking and memory function. Cognitive assessment tools evaluate the ability to learn and recall new information and measure changes in reasoning, problem-solving, planning, naming, comprehension and other cognitive skills.4 In the early stages of AD, cognitive assessment is the main method to diagnose AD; however, it is inaccurate in approximately 50% to 60% of patients.17 Blood work is also a standard procedure in the beginning stages of the diagnostic process.
In May 2022, the U.S. Food and Drug Administration (FDA) cleared the first in vitro diagnostic test to aid in the detection of AD. The Lumipulse G β-Amyloid Ratio 1-42/1-40 test detects amyloid plaques among those age 55 and older who are showing cognitive decline.18 During this test, cerebrospinal fluid is collected to determine the presence of amyloid plaques, similar to results revealed during a PET scan of the brain. Amyloid PET scans have been a standard tool for AD diagnosis, but this additional assay provides another option to identify the presence of amyloid plaques. Besides PET, other imaging techniques include magnetic resonance imaging and computed tomography to rule out other conditions in the brain.
Donepezil, rivastigmine, galantamine, memantine, and memantine combined with donepezil are medications available to treat the symptoms of AD.4 However, these medications do not alter the course of the disease. Aducanumab, the first drug aimed at treating the disease process, was approved by the FDA in June 2021. Aducanumab, which was studied in subjects with mild cognitive impairment and mild dementia due to AD, demonstrated the ability to reduce plaques in the brain. Controversy over this drug exists due to its limited ability to improve cognition and high cost. Other treatment options are aimed at improving quality of life, such as cognitive stimulation, music-based therapies, and cognitive behavioral therapy. These treatments may help to reduce behavioral symptoms, such as depression, apathy, wandering, sleep disturbances, agitation and aggression.4 Mood disorders can be treated with prescription medications, which may increase risk for xerostomia.
ADDRESSING PORPHYROMONAS GINGIVALIS AND GINGIPAIN INHIBITORS
Considering pathogenic oral bacteria increase the risk for AD, controlling these microorganisms should likely be part of the AD treatment protocol — and while such a protocol is not currently available, research is underway. Broad-spectrum antibiotics do not protect against P. gingivalis-induced cell death and do not clear P. gingivalis from the brain. Another clinical consideration is that P. gingivalis rapidly develops resistance to broad-spectrum antibiotics.9 Because gingipains help the organism thrive, directly targeting gingipain production may help control P. gingivalis. Orally administered gingipain inhibitors protect against brain cell death and clear P. gingivalis from the brain.9 Trials on this approach are ongoing. If this approach is successful, future research might show that treating periodontitis in the initial stages could affect the development or progression of AD.11
In patients who have a family history of AD, treating periodontitis at the earliest stages should be a priority. If a patient previously completed genetic testing for AD and is positive, periodontal treatment and maintenance should be emphasized to reduce the spread of pathogens within the body. Controlling biofilm growth and maturation can be accomplished through periodontal maintenance every 90 days, as well as daily oral care aimed at removing both supragingival and subgingival biofilm. The dental team should therefore seek to educate patients about the connection between oral health — or lack of it — and AD, and schedule dental visits accordingly.
CONCLUSION
With proper training, oral health professionals can directly observe behavioral signs related to AD in their patients. Individuals exhibiting AD-related symptoms should be referred to their primary care physician. Additionally, the presence of periodontitis should be evaluated closely and treated early.
References
- Alzheimer’s Association. What Is Dementia? Available at: https://www.alz.org/alzheimers-dementia/what-is-dementia. Accessed February 6, 2023.
- Freedman V, Cornman J, Kasper J. National Health and Aging Trends Study Trends Chart Book: Key Trends, Measures and Detailed Tables. Available at: https://micda.isr.umich.edu/wp-content/uploads/떖/葏/NHATS-Companion-Chartbook-to-Trends-Dashboards-2020.pdf. Accessed February 6, 2023.
- Rajan K, Weuve J, Barnes L, McAninch E, Wilson R, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17:1966–1975.
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures. Available at: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf. Accessed February 6, 2023.
- Abbayya K, Puthanakar N, Naduwinmani S, Chidambar Y. Association between periodontitis and Alzheimer’s disease. N Am J Med Sci. 2015;7:241–246.
- National Institute on Aging. Video: How Alzheimer’s Changes the Brain. Available at: https://www.nia.nih.gov/health/video-how-alzheimers-changes-brain. Accessed February 6, 2023.
- Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther. 2017;9:56.
- Alzheimer’s Association. Causes and Risk Factors for Alzheimer’s Disease. Available at: https://www.alz.org/alzheimers-dementia/what-is-alzheimers/causes-and-risk-factors. Accessed February 6, 2023.
- Dominy S, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333.
- The National Institute of Dental and Craniofacial Research. Periodontal Disease in Adults (Age 30 or Older). Available at: https://www.nidcr.nih.gov/research/data-statistics/periodontal-disease/adults. Accessed February 6, 2023.
- Ryder M. Porphyromonas gingivalis and Alzheimer’s disease: Recent findings and potential therapies. J Periodontol. 2020;91(Suppl 1):S45–S49.
- Positive Approach to Care. What is Dementia? Available at: https://teepasnow.com/about-dementia. Accessed February 6, 2023.
- Snow T. Workshop A: normal aging vs not normal aging. Available at: https://learn.teepasnow.com/trainer-post-certification-tools. Accessed February 6, 2023.
- Mendez MF. Degenerative dementias: Alterations of emotions and mood disorders. Handb Clin Neurol. 2021;183:261–281.
- Gonzalez Cademartori M, Torres Gastal M, Giacommelli Nascimento G, Fernando Demarco F, Britto Correa M. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin Oral Invest. 2018;22:2685–2702.
- Positive Approach to Care. The GEMS: Brain Change Model. Available at: https://teepasnow.com/about/about-teepa-snow/the-gems-brain-change-model/. Accessed February 6, 2023.
- Schneider J, Arvanitakis Z, Leurgans S, Bennet D. The neuropathology of probable Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208.
- Cassels C. FDA Clears diagnostic test for early Alzheimer’s. Available at: https://www.medscape.com/viewarticle/덋#:~:text=The%20US%20Food%20and%20Drug,of%20Alzheimer%E2%80%99s%20disease%20(AD). Accessed February 6, 2023.
From Decisions in Dentistry. March 2023;9(3)30-33.