
Oral Care Strategies for Patients with Head and Neck Cancers
Dental professionals are integral members of the interprofessional team required to manage patients undergoing cancer treatment as well as those who have recovered
This course was published in the November/December 2023 issue and expires December 2026. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 730
Educational Objectives
After reading this course, the participant should be able to:
- Discuss the interdisciplinary approach to head and neck squamous cell carcinoma (HNSCC) management.
- Identify early interventions for HNSCC.
- Explain the preventive dental strategies before, during, and after oncology treatment.
The diagnosis of head and neck squamous cell carcinoma (HNSCC) (Figure 1) can be devastating with many unknown outcomes. HNSCC is best managed with an interdisciplinary approach involving medical and radiation oncologists, primary care physicians, general dentists, dental hygienists, oral surgeons, maxillofacial prosthodontists, and dental oncologists. The literature demonstrates that the knowledge of oral health professionals on dental oncology is lacking.1
Dental clinicians are instrumental in the diagnosis of HNSCC with frequent oral cancer screening examinations. Patients may present with a variety of symptoms depending on the extent and location of the tumor. Nasal cavity and paranasal sinuses tumors have unilateral epistaxis or nasal obstruction. Nasopharyngeal and tonsillar tumors present late with nodal neck masses. Laryngeal tumors exhibit persistent hoarseness and oral cavity tumors display nonhealing ulcers, numbness, or pain.2
Once biopsied and diagnosed, the staging of the tumor and treatment approach are determined by the medical oncologist. Early-stage tumor treatment includes surgery or radiotherapy, which acts by damaging the DNA in highly proliferating cancer cells thereby ceasing replication. The 5-year survival rate for a stage I tumor is 80% to 90% whereas the survival rate drops to 65% to 80% with a stage II tumor.2
Once metastasis has occurred, resection surgery and chemotherapy may be options. Chemotherapy drugs are used in combination to avoid medication resistance.3 If the tumor is surgically unresectable, treatment with chemoradiation therapy, where chemotherapy is given at the same time as radiotherapy, may be considered. Chemoradiation therapy is the most effective in shrinking localized oral/oropharyngeal cancers.4 For late tumor stages, palliative care is given since the success rate drops to 10% to 40%.2
Two publications — The Dental Provider’s Oncology Pocket Guide for the Prevention and Management of Oral Complications in Head and Neck Radiation Therapy, Chemotherapy, and Hematopoietic Stem Cell Transplantation5 and The Dental Team: Oral Complications for Cancer Treatment What the Dental Team Can Do6 — outline the complex nature of cancer therapy, questions dentists should ask in interprofessional consultations, and the role that oral health professionals play in the management of oral sequelae.
Before Oncology Treatment
Prior to beginning oncology therapy, a comprehensive dental evaluation is recommended to eliminate potential oral sources of infection. Patients with good oral health tend to experience fewer complications.5,7,8
The comprehensive dental evaluation should be conducted at least 1 month before cancer treatment begins. In patients with hematologic cancers, immunosuppression, or thrombocytopenia, no dental procedures are performed without a consultation with the oncologist.5 The comprehensive dental evaluation includes a full-mouth series; panoramic radiograph; periodontal charting; and diagnosis of caries, periodontal diseases, and/or periapical pathosis. Dental treatment should strive to eliminate or arrest all oral disease.5 Sources of trauma and oral irritation should be addressed immediately. If time does not permit, then temporary restorations are acceptable. Final crowns or fillings can be completed after oncology therapy.9
At least 2 weeks before radiotherapy and 7 to 10 days prior to chemotherapy, the dentist may perform extractions or pre-prosthetic surgery. Depending on the oncologist’s recommendation, chlorhexidine and antibiotics may be required after extractions.5,9 Research shows that patients undergoing radiotherapy will experience a high caries rate and the risk of osteoradionecrosis may influence the treatment plan.10
All teeth should have pulpal vitality testing and any that test necrotic should be treated endodontically or extracted.3,10,11 Grossly decayed and nonrestorable teeth, those with severe periodontal disease and mobility, or partially erupted or symptomatic third molars should be removed. Elective oral surgery may be delayed until oncology therapy is concluded.5,9 Smoking promotes oral side effects with chemotherapy, so tobacco cessation counseling is advised.4 For radiotherapy, a strict 4-to-8-week hygiene recare program for the first 6 months after therapy should be scheduled.10
No removable dentures or partial dentures should be worn during radiotherapy or chemoradiation therapy due to mucositis and friable tissue damage. The dental hygienist should encourage optimal daily oral hygiene, including toothbrushing three times per day with an extra soft brush, daily flossing, and prescription fluoride use. Decreased salivary flow will necessitate prescription fluorides and custom gel-applicator trays for caries prevention. These additions will be a lifelong part of patients’ oral hygiene program.5,8,9
Facial anatomy and overall facial appearance may be altered after HNSCC surgery to the extent that a maxillofacial prosthodontist may be needed. The prosthodontist will determine if prosthetic rehabilitation can be done simultaneously with or after surgical treatment, the design of any prosthesis, and whether implants can be placed. Prosthetics can significantly improve function, diet, and quality of life for patients post-cancer treatment.12 Patients may also be referred to a dental oncologist. A dental oncologist is a dentist with fellowship training in the special care needs of patients with cancer.13 When dental services are complete, the cancer center may request a written dental clearance verifying that the comprehensive dental evaluation and treatment plan have been completed.9
During Oncology Treatment
Neck tumors, even with no evidence of nodal involvement, should be treated surgically. The tumor size, site, and proximity to bone influences the surgical approach. There are cases in which the surgeon may decide not to remove tissue due to esthetic or functional disabilities.14 In addition, tumor recurrence after surgery alone is 40% but in conjunction with chemoradiation therapy, the recurrence rate drops to 20%.2
The medical oncologist administers the chemoradiation therapy through intravenous central catheters, central venous access devices, or central lines.2 Chemoradiation therapy given before surgery is referred to as neoadjuvant chemoradiation therapy, which aims to decrease the size of the tumor to make it surgically more resectable. Neoadjuvant chemoradiation therapy is typically followed by chemoradiation therapy. Adjuvant chemoradiation therapy is done to eradicate tumor cells that may remain after neoadjuvant chemoradiation or after surgery. Both types of chemoradiation therapy are usually given over 3 to 6 months.15
Physical side effects of chemoradiation therapy include hair loss, loss of appetite or weight loss, nausea and vomiting, diarrhea, nail and skin changes, bruising, fatigue, and susceptibility to infection.4 Due to infection risks, no dental services, including prophylaxis, should be performed without the oncologist’s consent. A platelet and absolute neutrophil count must be performed at least 24 hours before invasive dental procedures are allowed. If there are abnormal clotting factors, no dental treatment should be performed. If the neutrophil count is low, then antibiotic prophylaxis may be advised, especially in patients with central venous catheters.5
The radiation oncologist delivers radiotherapy either alone or in combination with chemoradiation therapy and/or surgery. The typical dose for HNSCC ranges from 54 to 70 Gy. The radiation field may involve critical structures of the spinal cord, brainstem, optic pathways, brachial plexus, salivary glands, and larynx depending on the location and severity of the tumor.1 Intensity modulated and image-guided radiotherapy is used to target volumes more precisely, reduce irradiation dose, reducing harm to surrounding healthy tissues. This may reduce absorption by the parotid gland, which may decrease the risk of post-treatment xerostomia by 36%.16
The patient should present to the dental office every 4 to 6 weeks to review oral hygiene and monitor for oral complications of xerostomia, radiation caries, mucositis, trismus, infections, or inability to follow self-care instructions due to oral pain. Secondary infections from viruses are a concern with mucositis.5,8
After Oncology Treatment
After completion of therapy, the patient continues follow-up care with the medical and radiation oncologist, the primary care physician, and the dentist. Before beginning dental care, oral health professionals must obtain a medical consultation that confirms their hematologic status is normal and that immunosuppression is resolved.5
The primary care physician’s role is to educate cancer survivors about the signs and symptoms of cancer recurrence. The primary care physician and the dental team can be alert to signs and symptoms of recurrence, which include swelling in the head and neck region; persistent sore throat; persistent nasal obstruction or nose bleeds; difficulty breathing; numbness in the ear or jaw pain; difficulty chewing, swallowing, and moving the tongue and jaw; or blood in the saliva.
The primary care physician will also screen for secondary primary cancers. Up to 23% of survivors may develop secondary cancers in the lung or esophagus.17 With the oncology team, the primary care physician develops an individualized survivorship care plan. This includes follow-up scans, laboratory tests, and office visits along with information on late side-effects that patients can experience depending on the type of cancer and treatment received.17
The oral health professional’s role post-treatment is to identify and manage acute and chronic oral changes such as radiation caries, mucositis, xerostomia, osteoradionecrosis, periodontitis, trismus, oral candidiasis infections, dysgeusia, and medication-related osteonecrosis of the jaw (MRONJ).3,8,18
Patients undergoing radiotherapy for HNSCC are at a higher risk for dental caries due to radiation injury to the major and minor salivary glands, leading to hypofunction and xerostomia. Radiation damages the tooth structure itself. Almost 25% of patients undergoing radiotherapy and 21% of patients with chemoradiation therapy experience radiation caries.10 It is more aggressive than classical caries and involves nontraditional locations such as cusp tips and gumline. There is a higher incidence of recurrence of radiation caries so in-office fluorides, frequent routine follow-up visits, and optimal daily oral hygiene counseling are advised.18
Mucositis is the most common side effect of cancer treatment and affects 66% to 85% of patients.3,19 Mucositis occurs when reactive oxygen species cause DNA damage to proliferating mucosal cells.3 Severity increases with co-morbidities of chronic alcoholism, liver cirrhosis, and type I diabetes.19 Mucositis arises as early as 7 to 10 days after treatment begins and may be self-limiting 2 to 4 weeks after cessation.3 The consequences of mucositis are malnutrition, psychological/psychiatric symptoms, poor quality of life, and opportunistic oral infections. Dysphagia can cause additional symptoms of anorexia and psychological effects of anxiety and depression. If the patient is unable to consume meals, the oncologist may place a feeding tube to facilitate nutrition.19
The goals in managing mucositis are to alleviate oral pain and prevent secondary fungal or bacterial infections.8,19 Exacerbation of pain may be experienced with hot, spicy, or acidic foods and beverages, so these should be avoided. Topical anesthetics of benzocaine, lidocaine, dyclonine, and capsaicin may be advised.3 Additional recommendations include optimal daily oral hygiene, waterflosser, alcohol-free mouthrinse, chlorhexidine, or salt and baking soda in warm water.11 Lasers may manage pain and even prevent mucositis. Low-intensity laser wavelengths, when applied to stressed oral tissues, reduce cellular oxidative stress and inflammation.20
Xerostomia presents both during and after cancer therapy. A marked difference in salivary flow can be observed in as little as 2 weeks after the initial therapy. Damage to the salivary glands causes a more viscous saliva and an acidic environment in the oral cavity, with risk for caries and opportunistic fungal infections.18 Signs of diminished salivary flow include fissures at the commissures of the lip, difficulty swallowing or chewing food, and problems with speech. To encourage saliva production, frequent sips of water throughout the day, ice chips melted in the mouth, or sugarless lemon drops and xylitol gum may be beneficial.11 If there is remaining salivary gland function, then prescription of pilocarpine or cevimeline may improve salivary flow.3
Osteoradionecrosis may present within the first 3 years after cancer therapy is complete with an incidence rate of 8% to 10%, and can occur more than 10 years later. It is more commonly observed in the mandible due to a decrease in vascularization and increase in bone density. The risk factors for developing osteoradionecrosis include a dose of radiation larger than 60 Gy, poor oral hygiene, alcohol and tobacco use, and location and staging of the tumor.16
Defined as exposed bone that persists for more than 3 months or a radiopaque irregularity in the alveolar bone without the overlying mucosa being affected, osteoradionecrosis is often the result of tooth extraction (50%), therefore extractions should not be done during or after the patient receives radiation. Patients often experience an increase in aggressive radiation caries, therefore raising the need for post-treatment extractions.18 Schuurhuis et al21 noted that four out of 51 patients required at least one extraction within 2 years after radiotherapy. If tooth extraction must be performed, antibiotic prophylaxis, epinephrine-free local anesthetic, and hyperbaric oxygen therapy may be advised.5,10
Patients receiving radiotherapy for HNSCC show increased prevalence of periodontitis with loss of attachment and gingival recession due to changes in the oral microbiome flora and hyposalivation.18 Due to concerns regarding osteoradionecrosis, periodontal procedures, such as scaling and root planing and flap surgery, are contraindicated. If pain develops, antibiotics can be prescribed with hyperbaric oxygen therapy to improve healing. If osteoradionecrosis develops, dental prophylaxis and periodic oral examinations should be performed every 3 to 4 months to reduce the risk of infection.3
Trismus is most often due to post-radiation fibrosis or post-surgical scar formation. With sustained contraction of the muscles of mastication, trismus may limit jaw opening to less than 35 mm. To improve symptoms, opening and closing exercises performed three times per day or use of a mouth-opening device may be helpful.3,5
An opportunistic fungal infection, Candida albicans is responsible for oral candidiasis and most often occurs during and after radiotherapy in about 27% of patients.3 It may present as pseudomembranous candidiasis, erythematous candidiasis, and/or angular cheilitis. Nystatin lozenges or oral suspension may be prescribed.
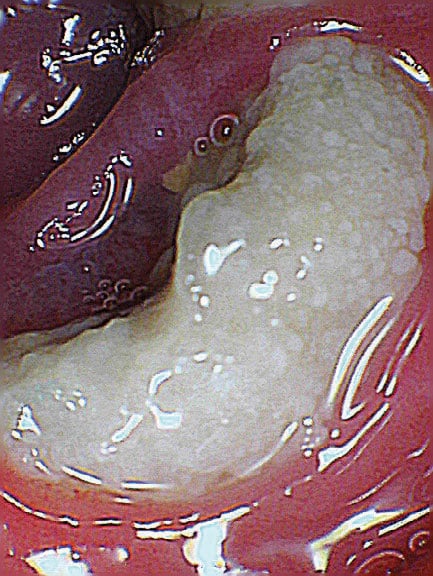
A change in taste, dysgeusia, can be noticed as early as the second week of radiotherapy. Typically, patients with HNSCC receive 2 Gy over the course of 6 to 7 weeks with a total of 60 to 72 Gy resulting in damage to the taste buds. However, 2 to 4 weeks after completion of radiotherapy, the taste sensations of bitter and acidic, which are most affected, may return.3
When metastasis occurs, intravenous bisphosphonate or a Rank-L inhibitor may be indicated. Both medications increase the risk of MRONJ.3 It is marked by persistent exposed bone in an individual with a background of using antiresorptive or antiangiogenic agents, without any history of radiation exposure to the head and neck area.
During stage 0, no exposed bone is seen and optimal daily oral hygiene is stressed. Stage 1 has exposed bone with no symptoms or infection. Stage 2 is symptomatic, with inflammation or infection, and necrotic exposed bone (Figure 2). Stage 3 has exposed necrotic bone with pain, infection, jaw fracture, and/or extraoral fistula and should be referred to an oral surgeon (Figure 3).3,22
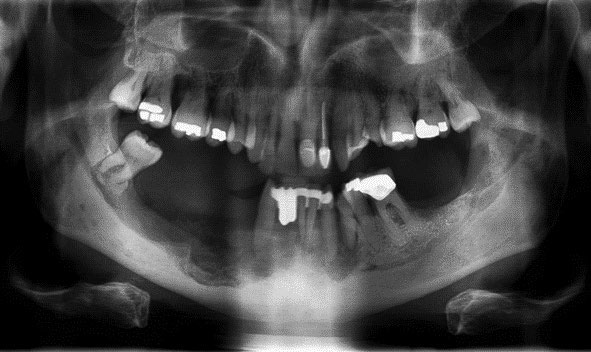
The 2022 American Association of Oral and Maxillofacial Surgeons position paper no longer recommends drug holidays or bone turnover markers. Patients being treated for HNSCC should consider root retention strategies for dental infections as opposed to oral surgery.22
Conclusion
Patients presenting with HNSCC require oncology treatment using an interdisciplinary approach between medical and dental professionals during all stages of treatment. Dental professionals need to manage the myriad of oral sequelae resulting from oncology therapy, increasing the patient’s overall survival rate and improving quality of life.
References
- Winter IP, Ingledew PA, Golden DW. Interprofessional education in radiation oncology. J Am Coll Radiol. 2019;16:964–971.
- Choong N, Vokes E. Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin. 2008;58:32–53.
- Kim RH, Yang P, Sung EC. Managing intraoral lesions in oral cancer patients in a general dental practice: an overview. J Calif Dent Assoc. 2016;44:85–92.
- American Cancer Society. Chemotherapy for Oral Cavity and Oropharyngeal Cancer. Available at: cancer.org/cancer/types/oral-cavity-and-oropharyngeal-cancer/treating/chemotherapy.html. Accessed November 14, 2023.
- National Institutes of Health. Dental Provider’s Oncology Pocket Guide. Available at: nidcr.nih.gov/sites/default/files/2017-09/oncology-guide-dental-provider_0.pdf. Accessed November 14, 2023.
- National Institutes of Health. Dental Team Oral Complications of Cancer Treatment: What the Dental Team Can Do. Available at: nidcr.nih.gov/sites/default/files/2020-06/oral-complications-cancer-dental-team.pdf. Accessed November 14, 2023.
- National Institutes of Health. Three Good Reasons to See a Dentist Before Cancer Treatment. Available at: nidcr.nih.gov/sites/default/files/2017-09/reasons-dentist-cancer-treatment-illustrations.pdf. Accessed November 14, 2023.
- Samim F, Epstein JB, Zumsteg ZS, Ho AS, Barasch A. Oral and dental health in head and neck cancer survivors. Cancers Head Neck. 2016;1:14.
- Dana-Farber Cancer Institute. Dental Guidelines for Patients Who May Need Radiation Therapy to the Head and Neck Area. Available at: dana-farber.org/uploadedFiles/Pages/For_Patients_and_Families/Care_and_Treatment/Treatment_Centers/Oral_Medicine_and_Dentistry/dental-radiation.pdf. Accessed November 14, 2023.
- Moore C, McLister C, Cardwell C, O’Neill C, Donnelly M, McKenna G. Dental caries following radiotherapy for head and neck cancer: a systematic review. Oral Oncol. 2020;100:104484.
- Joshi VK. Dental treatment planning and management for the mouth cancer patient. Oral Oncol. 2010;46:475–479.
- Vosselman N, Alberga J, Witjes MHJ, et al. Prosthodontic rehabilitation of head and neck cancer patients — challenges and new developments. Oral Dis. 2021;27:64–72.
- Harvard School of Dental Medicine. Oral Medicine and Oral Oncology Fellowship. Available at: hsdm.harvard.edu/oral-medicine-and-oral-oncology-fellowship. Accessed November 14, 2023.
- Mehta S, Kuriakose, MA. Principles of surgical management of oral cancer. In: Bonanthaya K, ed. Oral Maxofacial Surgery for the Clinician. New York: Springer; 2021.
- Gau M, Karabajakian A, Reverdy T, Neidhardt EM, Fayette J. Induction chemotherapy in head and neck cancers: Results and controversies. Oral Oncol. 2019;95:164–169.
- Alterio D, Marvaso G, Ferrari A, Volpe S, Orecchia R, Jereczek-Fossa BA. Modern radiotherapy for head and neck cancer. Semin Oncol. 2019;46:233–245.
- American Cancer Society. ASCO Cancer Treatment and Survivorship Care Plans. Available at: cancer.org/cancer/survivorship/long-term-health-concerns/survivorship-care-plans..html. Accessed November 14, 2023.
- Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918–2931.
- Shetty SS, Maruthi M, Dhara V, et al. Oral mucositis: current knowledge and future directions. Dis Mon. 2022;68:101300.
- Antunes HS, Herchenhorn D, Small IA, et al. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncology. 2017;71:11–15.
- Schuurhuis JM, Stokman MA, Witjes MJH, et al. Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: a 2-year prospective follow-up study. Support Care Cancer. 2018;26:1133–1142.
- Boyd B. Update on medication related osteonecrosis of the jaws. Dela J Public Health. 2023;9:42–43.
From Decisions in Dentistry.November/December 2023; 9(10):28-31




