
Immunizations for Oral Health Care Workers
The following vaccination strategies will help prevent disease transmission in the dental workplace.
This course was published in the August 2017 issue and expires August 2020. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be able to:
- Describe immunological response processes and how vaccination recommendations are formulated.
- List current immunization recommendations issued by the U.S. Centers for Disease Control and Prevention for health care professionals and their adult patients.
- Explain issues affecting compliance with immunization recommendations and vaccines.
The No. 1 public health achievement of the 20th century was the development of immunization to prevent communicable diseases. According to Molinari and Terezhalmy,1 vaccination (or immunization) is defined as “the act of artificially inducing immunity against a disease.” When discussing strategies for infection control, clinical staff must always refer to the universal chain of infection. Any method or strategy to break or block a step in the chain of infection will help prevent or reduce the risk of disease transmission. Vaccinations administered with the prescribed dose and series of injections will usually elicit a host immunological response that renders the host unsusceptible to disease.
The host response of an individual’s functioning immune status is a critical determinant in preventing disease. An adult acquires immunity by (1) developing symptomatic or asymptomatic infection and recovering, with their immune system actively producing an immune response (natural active immunity); (2) receiving injections of antibodies from an immune individual to prevent the onset of infection (artificial passive immunity); or (3) administering a specific antigen and having the immune system stimulated to produce an immune response without manifesting disease (artificial active immunity).1
The purpose of this article is to explain immunization strategies that will help prevent disease transmission in the dental workplace. It will also provide the latest immunization recommendations for dental health care personnel, as issued by the U.S. Centers for Disease Control and Prevention (CDC), and discuss updated scientific and clinical knowledge concerning current vaccine recommendations for adult patients.
UPDATED RECOMMENDATIONS
The CDC’s Advisory Committee on Immunization Practices (ACIP) is a group of medical and public health professionals that develops recommendations on how to use vaccines to control diseases in the United States. It consists of 15 experts who are voting members and are responsible for making vaccine recommendations. Fourteen members have expertise in vaccinology, immunology, pediatrics, internal medicine, nursing, family medicine, virology, public health, infectious diseases and/or preventive medicine. One member is a consumer representative who provides perspectives on the social and community aspects of vaccination. In addition, ACIP works with professional groups, including the American Academy of Pediatrics and American Academy of Family Physicians. In October 2016, the advisory committee voted to approve the latest Recommended Adult Immunization Schedule for Adults Aged 19 Years or Older — United States, 2017. These recommendations were posted on the CDC’s website in February 2017 as a Morbidity and Mortality Weekly Report Early Release.2 The 2017 adult immunization schedule was also reviewed and approved by the American College of Physicians, American Academy of Family Physicians, American College of Obstetricians and Gynecologists and the American College of Nurse Midwives.
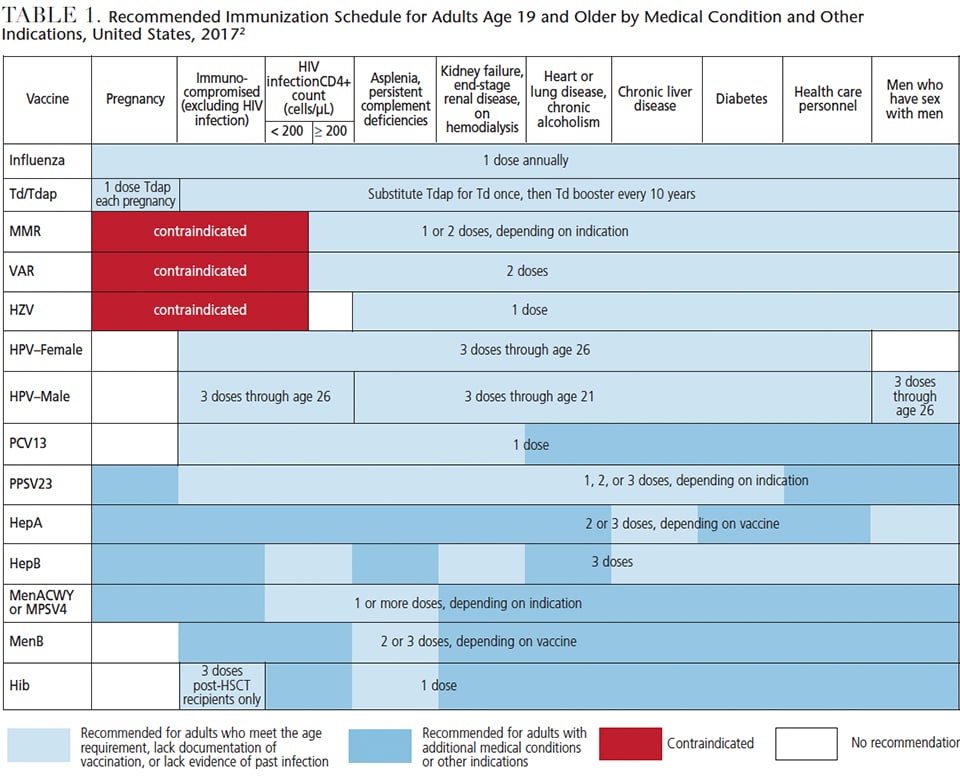
The 2017 ACIP Adult Immunization Schedule (Table 1) lists the recommended immunizations for all health care personnel: influenza, Td/Tdap (for tetanus, diphtheria and pertussis), MMR (for measles, mumps and rubella), VAR (for varicella), HZV (for zoster), HPV/male and female (for human papillomavirus), and HepB (for hepatitis B).2 Note that the other vaccinations listed in Table 1 are specific for adults (age 19 and older) with additional medical conditions or other indications, and these vaccinations are for pneumonia, hepatitis A, Haemophilus influenza type b infection and meningococcal disease. Changes in the 2017 adult immunization schedule from the previous year’s schedule include new or revised ACIP recommendations for influenza, human papillomavirus, hepatitis B and meningococcal vaccines.2
Influenza Vaccinations: The live attenuated influenza vaccine or nasal spray are not recommended and should not be used due to low effectiveness. In addition, adults with a history of egg allergy who have only hives after exposure to egg should receive age-appropriate inactivated influenza vaccine (IIV) or recombinant influenza vaccine (RIV), while adults with a history of egg allergy with symptoms other than hives (e.g., angioedema, respiratory distress, lightheadedness or recurrent emesis, or who required epinephrine or alternate emergency medical intervention) may receive age-appropriate IIV or RIV. The selected vaccine should be administered in an inpatient or outpatient medical setting and supervised by a health care provider who is capable of recognizing and managing severe allergic reactions.
Human Papillomavirus Vaccinations: Healthy adolescents who start their HPV vaccine series before age 15 are recommended to receive two doses of HPV. However, the recommendation remains three doses for adults and adolescents who did not start their vaccination series before age 15. Changes in recommendations in the adult immunization schedule include updates regarding HPV vaccination for adults who did not complete HPV series as adolescents.
These changes are described in the 2017 adult immunization schedule as:
- Women through age 26 and men through age 21 who have not had any HPV should receive a three-dose series of HPV at 0, 1 to 2, and 6 months
- Men age 22 through 26 may be vaccinated with a three-dose series of HPV at 0, 1 to 2, and 6 months
- Women through age 26 and men through age 21 (as well as men ages 22 through 26 who may receive HPV) who initiated the HPV series before age 15 and received two doses at least 5 months apart are considered adequately vaccinated and do not need an additional dose of HPV
- Women through age 26 and adult males through age 21 (as well as men ages 22 through 26 who may receive HPV) who initiated the series before age 15 and received only one dose, or two doses less than 5 months apart, are not considered adequately vaccinated and should receive one additional dose of HPV
Hepatitis B Vaccinations: The ACIP has updated its list of chronic liver disease conditions for which a HepB series vaccine is recommended. This change is described in the 2017 adult immunization schedule as: Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, autoimmune hepatitis, and an alanine aminotransferase or aspartate aminotransferase level greater than twice the upper limit of normal, should receive a HepB series.
Meningococcal Vaccinations: Two changes in meningococcal vaccination recommendations were issued in 2017. First, ACIP recommends that adults with human immunodeficiency virus (HIV) infection should receive a two-dose primary series of serogroups A, C, W and Y meningococcal conjugate vaccine (MenACWY). Second, ACIP provides updated dosing guidance for one of the serogroup B meningococcal vaccine (MenB) — MenB-FHbp (Trumenba, Pfizer). For adults who are at increased risk for meningococcal disease and for use during serogroup B meningococcal disease outbreaks, three doses of MenB-FHbp should be administered at 0, 1–2, and 6 months. When MenB-FHbp is given to healthy adolescents and young adults who are not at increased risk for meningococcal disease, two doses of MenB-FHbp should be administered at 0 and 6 months. Note that the dosing frequency and interval for the other MenB, MenB-4C (Bexsero, GlaxoSmithKline) have not changed; MenB-4,C remains a two-dose series, with doses administered at least a month apart. Either MenB can be used when indicated. The change to ACIP recommendations on the use of MenB=FHbp does not imply a preference for one MenB over the other. These updates in meningococcal vaccinations are reflected in the 2017 adult immunization schedule as:
- Adults with anatomical or functional splenia or persistent complement component deficiencies should receive a two-dose primary series of MnACWY at least 2 months apart and revaccinate every 5 years. These individuals should also receive a series of MenB with either a two-dose series of MenB-4C at least a month apart, or a three-dose series of MenB-FHbp at 0, 1 to 2, and 6 months.
- Adults with HIV infection who have not previously been vaccinated should receive a two-dose primary series of MenACWY at least 2 months apart and revaccinate every 5 years. Those who previously received one dose of MenACWY should receive a second dose at least 2 months after the first dose. Adults with HIV infection are not routinely recommended to receive MenB because meningococcal disease in this population is caused primarily by serogroups C, W and Y.
- Microbiologists who are routinely exposed to isolates of Neisseria meningitidis should receive one dose of MenACWY and revaccinate every 5 years if the risk for infection remains, and either a two-dose series of MenB-4C at least a month apart, or a three-dose series of MenB-FHbp at 0, 1 to 2, and 6 months.
- Adults at risk because of a meningococcal disease outbreak should receive one dose of MenACWY if the outbreak is attributable to serogroup A, C, W or Y, or either a two-dose series of MenB-4C at least a month apart, or a three-dose series of menB-GHbp at 0, 1 to 2, and 6 months if the outbreak is attributable to serogroup B.
- Young adults ages 16 through 23 years (the preferred age group is 16 through 18 years) who are healthy and not at increased risk for serogroup B meningococcal disease may receive either a two-dose series of MenB-4C at least a month apart, or a two-dose series of MenB-FHbp at 0 and 6 months for short-term protection against most strains of serogroup B meningococcal disease.
IMPORTANCE OF IMMUNIZATION
While these detailed dosage and administration recommendations may seem overwhelmingly complex to oral health care providers, dental teams are advised to familiarize themselves with the general changes regarding the four specific recommendations. Indeed, even primary care physicians report a high level of confusion regarding the adult vaccination schedule. A study found that 29% of general internists and family physicians reported confusion surrounding some aspects of ACIP’s vaccine schedule. In addition, 25% of the study respondents agreed the age-based indications for immunizations are difficult to follow, and 29% agreed that medical condition-based indications are difficult to follow.3 According to the study, general internists were less likely than family physicians to report that ACIP’s vaccine schedule provides clear guidance on what to do about catch-up vaccines.3
To make it easier for clinicians to access the vaccine schedules, a free CDC Vaccine Schedules App is available, and though optimized for tablets, it is also useful on smartphones. The app shows the child, adolescent and adult vaccines recommended by ACIP and visually mimics the printed schedules (for more information, visit cdc.gov/vaccines/schedules/hcp/schedule-app.html).
Dental professionals should recognize the importance of immunization in preventing disease, and educate staff and patients about the health advantages of the vaccinations recommended by the CDC and ACIP. Clinicians should educate themselves first and then patients in order to deliver accurate information regarding the benefits for adults and children. But the challenge really comes down to each provider’s beliefs and actions in terms of being vaccinated and making sure the recommendations are followed. By “walking the talk,” clinicians who keep their immunizations up to date send a powerful message to team members and patients to do the same and get vaccinated. They should also be aware of Occupational Safety and Health Administration requirements regarding hepatitis B virus immunization for health care workers.

so they can effectively advise patients about their importance in preventing disease.Image by YAKOBCHUKOLENA/ISTOCK/GETTY IMAGES PLUS
If clinicians examine the reported data and research regarding influenza vaccination coverage and compliance among health care personnel in the United States, the results are clearly suboptimal. A CDC Morbidity and Mortality Weekly Report issued September 30, 2016 discussed the findings of a CDC Internet panel survey that provided estimates of influenza vaccination coverage among health care personnel during the 2015–2016 influenza season. Of the 2316 health care personnel who completed the survey, 79.0% of respondents reported having received an influenza vaccination during the 2015–2016 season. While this represented a 15.5% increase compared to the 2010–2011 season estimate, it was similar to the 77.3% estimate reported in 2014–2015 season. The survey shows that coverage was highest among physicians (95.6%) and lowest among assistants and aides (64.1%). Among vaccinated health care personnel, 72.7% were vaccinated at their workplace. As it stands to reason, employer vaccination requirements and offering vaccination at the workplace at no cost were associated with higher vaccination coverage. As stated by CDC, “The implications for public health practice is that implementing comprehensive, evidence-based worksite intervention strategies is important to ensuring health care personnel and patients are protected against influenza.”4
What about dental health care and vaccination coverage results? A 2012 study entitled “Attitudes of Dental Health Care Workers Toward the Influenza Vaccination” questioned students and staff of a German dental university about their acceptance or declination of the influenza vaccination. “The main reason for not getting vaccinated against the pandemic influenza A/H1N1 virus in the 2009–2010 season was the objection to the AS03 adjuvants (48.5%). The authors concluded, “Our findings confirm the importance of a comprehensive approach to the influenza vaccination, ensuring that dental health care personnel are correctly informed about the vaccine, and that it is convenient to receive it. It could be shown that an immunization campaign at the workplace seems to be capable of improving vaccination rates.”5
Adjuvants and substances are added to certain vaccines to increase the body’s immune response to the vaccine; these may be introduced to inactivate viruses or bacteria, to preserve them and/or prevent them from losing potency over time. Enhanced vaccines are tested for safety in clinical trials before they are licensed for use in the United States. Through its Immunization Action Coalition, the CDC has developed an information page on adjuvants and ingredients resources.6 It is important to note that, like any medicine, vaccines can cause minor side effects that may include slight fever, rash or soreness at the site of injection. Slight discomfort is normal and should not be cause for alarm. It is extremely rare that a vaccine causes serious reactions. If a serious reaction occurs — and in consultation with a physician or health department — dental providers may file a Vaccine Adverse Event Reporting System report (for more information, visit https://vaers.hhs.gov/index).
NEED FOR EDUCATION
As noted, oral health care providers should educate team members, patients and especially parents and caregivers about the need for immunizations, and why individuals should not wait to vaccinate. Children younger than age 5 are susceptible to disease because their immune systems have not built up the necessary defenses to fight infection. By immunizing children as recommended (by age 2), parents and caregivers can protect them from disease and protect others at school or daycare. Why should children be vaccinated? Immunizations protect children from childhood diseases that can cause serious complications and death, including diphtheria, influenza, Haemophilus influenzae type b, hepatitis A, hepatitis B, HPV disease, measles, meningococcal disease, mumps, pertussis, pneumonia, polio, rotavirus disease, rubella, tetanus and chickenpox.
Dental teams must help influence and educate patients about this topic. By way of example, the New York Times recently covered the largest measles outbreak in Minnesota in three decades.7 As of presstime, Minnesota had reported 44 confirmed cases since April 2017 — the largest outbreak this year in the United States. Of note, measles was nearly eradicated in the United States before research in 2000 — later discredited — stoked fears of a link between vaccines and autism.
With the upsurge of measles cases in the U.S., health care providers understand that vaccine-preventable diseases still carry a significant risk of resurgence. If the U.S. population stopped routine vaccination of children, diseases that are almost unknown today could possibly stage a comeback. Under this scenario, epidemics of diseases that are nearly under control today could emerge. As history has shown with smallpox — a disease that was eradicated — U.S. children don’t have to receive smallpox shots anymore. Smallpox is now only a memory. If health care professionals, parents and caregivers keep vaccinating, it is possible to eliminate diseases that threaten children and adults. In short, vaccinations are crucial to protecting Americans’ future herd immunity.
REFERENCES
- Molinari JA, Terezhalmy GT. Immunizations for dental health care personnel. In: Molinari JA, Harte, JA, eds. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:89–100.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older — United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:136–138.
- Hurley LP, Bridges CB, Harpaz R, et al. Physician attitudes toward adult vaccines and other preventive practices, United States, 2012. Public Health Rep. 2016;131:320–330.
- Black CL, Yue X, Ball SW, et al. Influenza vaccination coverage among health care personnel — United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2016;65:1026–1031.
- Wicker S, Rabenau HF, Betz W, Lauer HC. Attitudes of dental health care workers toward the influenza vaccination. Int J Hyg Environ Health. 2012;215:482–486.
- U.S. Centers for Disease Control and Prevention: Immunization Action Coalition. Adjuvants and Ingredients Resources. Available at: immunize.org/talking-about-vaccines/adjuvants.asp. Accessed June 29, 2017.
- Mele C. Minnesota Sees Largest Outbreak of Measles in Almost 30 Years. New York Times. May 5, 2017. Available at: nyti.ms/2pe5JD9. Accessed June 29, 2017.
Featured Image by SCHWARTZ/E+/GETTY IMAGES PLUS
From Decisions in Dentistry. August 2017;3(8):40–44.





Please be sure to mention the National Childhood Vaccine Injury Act of 1986 that protects pharmaceutical companies from liability when their vaccines cause debilitating injuries and death. Thank you.