 FOTOSTORM/ISTOCK/GETTY IMAGES PLUS
FOTOSTORM/ISTOCK/GETTY IMAGES PLUS
Diagnosis, Management and Prevention Of Dentinal Hypersensitivity
Understanding the etiology of dentinal hypersensitivity can help practitioners implement patient-specific preventive and therapeutic measures.
This course was published in the June 2020 issue and expires June 2023. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the prevalence of dentinal hypersensitivity and stimuli that can elicit a painful response.
- Explain treatments designed to provide relief to patients with sensitivity.
- Describe optimal approaches to the prevention and
management of hypersensitivity
INTRODUCTION
How many patients do you treat for dentinal hypersensitivity? Do you have patients who experience sensitivity, but don’t tell you because they either believe sensitivity is normal, or they are afraid of restorative treatment to manage it? Or perhaps they think because it does not hurt all the time and if they don’t brush, it feels better, they can live with it. These are silent sufferers.
These patients will avoid activities, shun ice cream, and skip brushing areas that elicit pain. Of course, not brushing sensitive areas will only lead to more disease and more pain. The truth is, dentinal hypersensitivity is easy to treat once it is diagnosed. This article will help you diagnose hypersensitivity and provide immediate and effective treatment. It will also assist you in managing this condition to prevent further recession that can contribute to sensitivity issues.
Colgate Oral Pharmaceuticals is pleased to sponsor this CE article, “Diagnosis, Management and Prevention of Dentinal Hypersensitivity” to support you as you care for your silent sufferers.
—Phyllis Martina, RDH, MBA
Senior Professional Education Manager
Colgate Oral Pharmaceuticals
A Proper Diagnosis and Individually Tailored Therapy Can Help Prevent and Treat Dentinal Hypersensitivity
Dentinal hypersensitivity is defined as a sharp, painful response to external stimuli applied to exposed dentin that cannot be attributed to other dental or systemic sources.1 Long-term dentinal hypersensitivity is common; affecting approximately 11.5% of patients,2 it can have significant impact on quality of life,3 causing alterations in food choices, oral hygiene, and even time spent outdoors.4 Despite these consequences, it is estimated that 50% of patients who experience hypersensitivity do not report their pain.5 Possible causes that may expose dentin, and ultimately lead to sensitivity, include toothbrush abrasion, acid erosion, anatomical factors, gingival recession and periodontal involvement.4,6–8 To ensure optimal care, proper identification of gingival recession and dentinal hypersensitivity, adequate management, and effective preventive measures are critical.4,7
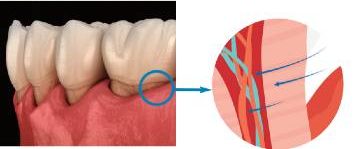
Anatomically, the interdental pulp is lined by odontoblast cells and the dentin is penetrated by dentinal tubules, typically 0.5 to 2 μm in diameter, into which the odontoblastic processes extend. These processes are suspended in a plasma-like fluid, and it is believed that movement of this fluid — known as hydrodynamic theory — can trigger mechanoreceptors present on the odontoblasts and further stimulate pulpal nerves, specifically A-β and A-δ myelinated fibers. This may elicit short, intense pain that may be induced by tactile, thermal and/or chemical stimuli.6,7
Two conditions must be met for sensitivity to develop: exposed dentin must be present (lesion localization), and open dentinal tubules communicating with vital pulp must be exposed to stimuli (lesion initiation).7 Histologic findings support this theory and demonstrate greater numbers of widened dentinal tubules in sensitive versus nonsensitive teeth9 (Figure 1). Consequently, many treatment modalities focus on complete or partial occlusion of tubules to reduce fluid flow and, thus, pain.
HYPERSENSITIVITY DIAGNOSIS
Diagnosis of dentinal hypersensitivity is challenging as patient self-reporting may be unreliable. In population studies, self-reported hypersensitivity rates differed significantly from those determined by clinical testing.10,11 Practically, clinical diagnosis is often an elimination diagnosis.4,12 Other sources of sensitivity/pain — including chipped or cracked teeth, fractured/faulty restorations, caries, gingival inflammation, hypoplastic enamel, a congenitally open cementoenamel junction, post-restorative sensitivity, occlusal trauma and pulpitis — must also be eliminated.13 A thorough medical and dental history and a comprehensive clinical and radiographic examination to rule out alternative causes of pain should be performed. Gingival recession, including a historical record of recession to determine if new dentin has become exposed, should be recorded.
Screening questions should include: (1) Do you avoid any foods, beverages or activities due to sensitivity in your teeth? (2) Do you avoid brushing or flossing due to sensitivity? (3) Did anything we did at your appointment cause sensitivity? If patients respond in the affirmative, further assessment is warranted.
If possible sensitivity is identified, a review of the presenting symptoms, including a history of symptoms and response to stimuli, should be performed. Generally, providers will use cold, an air blast or tactile stimuli to elicit a response.14 This testing can help establish a diagnosis and characterization of severity.15,16 Applying a systematic approach to screening and diagnosis is critical and allows for more targeted, personalized therapies.
Since permeability and fluid movement in open/exposed tubules is thought to be the cause of sensitivity, principles of treatment include reducing or eliminating irritating stimuli, tubule obliteration (i.e., dentinal plugs), and tubule coverage .2,3,6,17
Acid exposure has been found to be associated with both prevalence and severity of dentinal hypersensitivity.3,18 Individualized oral hygiene instructions should be a component of standard dental therapy. Dietary counseling to limit irritating foods and beverages can also be considered, particularly for patients who may be at risk for root sensitivity.
DENTIFRICES AND SELF-CARE PRODUCTS
Treatment with dentifrices and/or self-care products is often a first-line approach and may be combined with in-office therapies. The ingredients in these products either reduce nerve conduction or occlude dentinal tubules. Since these agents may utilize different mechanisms, patients may need to try several therapies or a combination to find relief.
Potassium Nitrate — Potassium nitrate is believed to depolarize nerves within the dentinal tubules and may inhibit or reduce their ability to transmit pain signals.19 The efficacy of potassium nitrate dentifrice is equivocal: While some studies have demonstrated symptom reduction over two weeks, others, including a Cochrane Database systematic review, have demonstrated no statistically significant changes with typical use.19 Despite these findings, potassium nitrate has not been shown to induce any deleterious pulpal changes, and may still have benefit in some patients.20
Strontium Salts — Strontium in toothpastes is designed to occlude dentinal tubules.21 Strontium ions exchange with calcium ions, causing the formation of insoluble strontium crystals within exposed tubules up to an average depth of 5 µm.22 Clinical studies have demonstrated the efficacy of strontium salt-containing dentifrice on sensitivity in patients.22
Arginine and Calcium Carbonate — Arginine is an amino acid that has a positive charge at physiologic pH. When combined with a bicarbonate buffer and calcium carbonate, it precipitates into exposed dentinal tubules. The positive charge of the calcium carbonate-arginine complex facilitates precipitation on the negatively charged dentinal surface.23 Additionally, the alkalinity of the arginine and calcium carbonate results in increased uptake of calcium and phosphate ions into saliva, which are then deposited on dentin.23 The reduction in sensitivity noted with arginine and calcium carbonate persists for up to 28 days.23,24 Patients using an arginine dentifrice demonstrated significant reductions in sensitivity symptoms at 24 weeks,25 and the combination of arginine and calcium carbonate resulted in improved reduction of sensitivity when compared with strontium acetate.26 Additionally, a novel formulation for at-home application is currently available. This serum may be used as a primary or adjunctive therapy (Colgate Anywhere, Anytime Sensitivity Relief System).
Calcium Sodium Phosphosilicate — In the oral cavity, calcium sodium phosphosilicate exchanges sodium ions with hydrogen ions, inducing the release of calcium and phosphate within the toothpaste. The calcium and phosphate minerals can then deposit within dentinal tubules, resulting in occlusion and relief.27,28
Fluoride — Both stannous and sodium fluoride are effective in managing sensitivity.29,30 These are usually prescription-only fluoride toothpastes/gels that can deliver 5000 to 12,5000 ppm fluoride. Their therapeutic effects may be due to both an increase in the occlusion of tubules and a reduction in acid decalcifications.31 Fluoride has an additional advantage of remineralizing dental tissues and reducing susceptibility to radicular caries. This may be of increased importance for patients with gingival recession and resultant root sensitivity, who may have an increased risk for caries.32
PROFESSIONALLY APPLIED DESENSITIZERS
Dentin desensitizers are used to treat sensitivity in an in-office setting, and are often used in combination with patient-delivered desensitizers and/or other therapies. They may contain fluoride, arginine and calcium carbonate, bonding agents, glutaraldehyde, oxalate, and casein phosphopeptide/amorphous calcium phosphate.
Fluoride — Fluoride varnish is commonly applied to exposed, symptomatic root surfaces. The varnish interacts with saliva, which increases its stability on the tooth surface, allowing increased fluoride uptake. Application of varnish has been shown to reduce sensitivity for up to 24 weeks, and has a further benefit of reducing risk for radicular caries.33
Arginine and Calcium Carbonate — Arginine/calcium carbonate is available in a paste that can be applied to exposed root surfaces and in a serum for both professional and self-care application. Evidence suggests patients experience an immediate reduction in sensitivity symptoms when arginine is applied as a desensitizer.24,34 A systematic review confirms the effectiveness of professionally applied arginine and calcium carbonate.35
Bonding Agents — Methacrylate resins and self-etch bonding systems — containing both acidic ingredients to condition the dentin and monomers that cover dentinal tubules — have been used as desensitizers.36 In general, these resins provide long-lasting relief,37 but the return of sensitivity is likely when the resin breaks away, reexposing tubules. This technique may be best used for recalcitrant cases of localized sensitivity, rather than generalized dentinal pain.38
Glutaraldehyde — Used alone or in combination with hydroxyethyl methacrylate (HEMA), glutaraldehyde serves as a desensitizing varnish. In this case, HEMA acts as a wetting agent to allow for better adhesion of the glutaraldehyde to the tooth surface.39,40 These agents penetrate up to 50 to 200 µm and can occlude dentinal tubules.40 Modest to moderate reduction in sensitivity has been reported with glutaraldehyde alone, but the addition of HEMA increases efficacy and may prolong effects up to nine months.41
Oxalate — Oxalate is a professionally applied desensitizer that combines with calcium ions in saliva to form insoluble calcium oxalate crystals that precipitate within dentinal tubules.37,42 Acid etching prior to application may allow for deeper penetration of the crystals.42 Oxalate has been shown to reduce sensitivity and to be stable in an acidic environment.37
Casein Phosphopeptide/Amorphous Calcium Phosphate — Casein-derived peptides bind to exposed dentin surfaces and allow for deposition of minerals from salivary and other oral fluids on these surfaces, reducing tubule patency. Bioavailable calcium and phosphate are deposited, which can be released during intraoral acidic challenges and may encourage remineralization, but efficacy studies are equivocal.43
ADVANCED TECHNOLOGIES
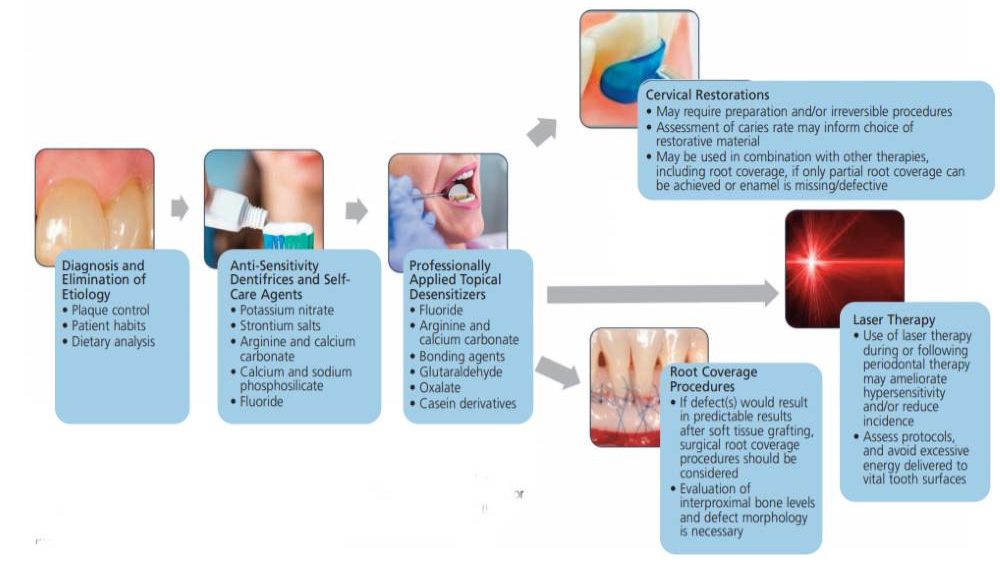
Clinicians can also select alternative therapies, including experimental desensitizers, lasers, cervical restorations and root coverage procedures.
Dendrimers — A novel experimental desensitizer synthesized using fluorohydroxyapatite crystals as a filler for poly(DMA-co-MEA) polymer may have the potential to release calcium, phosphate and fluoride at acidic pH.44 Additionally, dendrimers — artificial proteins with large branches — may be linked with antimicrobial and/or anti-inflammatory agents and could control pulpal inflammation or odontoblast irritation and reduce pain.45 Dendrimers could also potentially be combined with calcium, phosphate and/or fluoride, which might be released over time, allowing the minerals to enter the dentinal tubules and calcify to create a permanent barrier to fluid movement.45
Laser Therapy — Middle/high-output lasers (e.g., neodymium-doped yttrium aluminum garnet [Nd:YAG], erbium yttrium aluminum garnet [Er:YAG], diode and carbon dioxide) have been studied for use in managing dentinal hypersensitivity by occluding dentinal tubules.6,46 These lasers, at the wavelengths and protocols tested, have not been shown to permanently alter dentin surfaces through melting the dentin. Rather, it is believed they may reduce sensitivity through increased coagulation of proteins, which precipitate to block the tubules and hamper fluid flow.6,46 Lower-level lasers (e.g., helium-neon and gallium-aluminum-arsenate) may also affect nerve activity.46 Recent studies show Nd:YAG lasers may result in superior occlusion of dentinal tubules, but current evidence of comparative effectiveness and cost-utility analyses are lacking.46 Additionally, some laser therapies (in particular, lower-level lasers) may require multiple applications to achieve clinically significant results.46 While laser therapy shows promise, further research to determine the optimal clinical scenarios is needed.
Cervical Restorations — Cervical restorations cover dentinal tubules with restorative resins that are more extensive than bonding agents, but may require irreversible preparation of tooth structure to achieve retention. Marginal overhangs and leakage can predispose sites to plaque, recurrent caries and/or gingival inflammation. For patients with high radicular caries risk, the use of glass ionomer (with fluoride release) may mitigate the risk of caries.47 Overall, cervical restorations should be considered only after failure of less invasive treatments, and in cases in which gingival grafting is likely to be unsuccessful.
Periodontal Root Coverage Procedures — Soft tissue grafting can be effective in reducing sensitivity at sites with gingival recession. These results may persist longer than topical applications and also result in increased periodontal root coverage and potential alterations of periodontal phenotype — inuring sites from future gingival recession.48 Grafting is most successful in areas with buccal gingival recession, and the predictability of root coverage depends upon levels of interproximal bone and other anatomic factors.48 While similar reductions in dentinal hypersensitivity are seen with cervical restorations and gingival root coverage grafts, patients preferred gingival grafting for esthetic reasons.3,8
Because soft tissue root coverage procedures are effective and long lasting in reducing dentinal hypersensitivity, their use at suitable sites should be considered. Careful assessment to determine the likelihood of good prognoses for soft tissue grafting — based on the patient’s periodontal phenotype, interdental bone and attachment levels, and gingival recession morphology — is critical.49
CONCLUSION
Dentinal hypersensitivity is a common occurrence and often underreported.5,7 Targeted screening and proper diagnosis will help improve patients’ quality of life and allow them to better tolerate dental procedures. This review presents treatment options that range from noninvasive to irreversible. Optimal treatment utilizes a systematic approach to identify appropriate therapies based upon proper diagnosis, assessment of severity, and underlying etiologies. It is beneficial to weigh the advantages and disadvantages of each therapeutic approach, and provide personalized care that focuses on less invasive, first-line therapies before attempting more invasive treatment (Figure 2).
REFERENCES
- Holland GR, Nähri MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity J Clin Periodontol. 1997;24:808–813.
- Idon PI, Sotunde OA, Ogundare TO. Beyond the relief of pain: dentin hypersensitivity and oral health-related quality of life. Front Dent. 2019;16:325–334.
- Felix J, Ouanounou A. Dentin hypersensitivity: Etiology, diagnosis, and management. Compend Contin Educ Dent. 2019;40:653–657.
- Zeola LF, Soares PV, Cunha–Cruz J. Prevalence of dentin hypersensitivity: systematic review and meta-analysis. J Dent. 2019;81:1–6.
- Colgate-Palmolive Market research through Zapera. Data on file, 2009.
- Arahan AC, Pimenta LA, Marchi GM. Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res. 2009;23:333–339.
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367–375.
- de Oliveira DW, Marques DP, Aguiar-Cantuária IC, Flecha OD, Gonçalves PF. Effect of surgical defect coverage on cervical dentin hypersensitivity and quality of life. J Periodontol. 2013;84:768–775.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284.
- Graf H, Galasse R. Morbidity, prevalence, and intraoral distribution of hypersensitive teeth. J Dent Res. 1977;53(Spec Issue A):A162.
- Gilliam DG, Seo HS, Newman HN, Bulman JS. Comparison of dentine hypersensitivity in selected occidental and oriental populations. J Oral Rehab. 2001;28:20–25.
- Turp JC. Disucssion: how can we improve diagnosis of dentin hypersensitivity in the dental office? Clin Oral Investig. 2013;17(Suppl 1):S53–S54.
- Blaggana A, Vohra P, Nagpal A. Diagnosis and treatment of dentinal hypersensitivity. J Innovative Dent. 2011;3:1–4.
- Amarasena N, Spenser J, Ou Y, Brennan D. Dentine hypersensitivity in a private practice patient population in Australia. J Oral Rehabil. 2011;38:52–60.
- Gernhardt CR. How valid and applicable are current diagnostic criteria and assessment methods for dentin hypersensitivity? An overview. Clin Oral Investig. 2013;17(Suppl 1):S31–S40.
- Garcia-Godoy F, Trushkowsky R. A diagnostic device to record dentin hypersensitivity. American J Dent. 2013;26(Spec No B):3B–4B.
- Kim S, Kim E, Kim D, Lee I. The evaluation of dentinal tubule occlusion by desensitizing agents: A real-time measurement of dentinal tubule flow rate and scanning electron microscopy. Oper Dent. 2013;38:419–428.
- Addy M. Mostafa P, Newcombe G. Dentine hypersensitivity: the distribution of recession, sensitivity, and plaque. J Dent. 1987;15:242–248.
- Poulsen S, Errboe M, Hovgaard O, Worthington HW. Potassium nitrate toothpaste for dentine hypersensitivity. Cochrane Database Syst Rev. 2001;2:CD001476.
- Tarbet WJ, Buckner A, Stark MM, Fratarcangelo PA, Augsburger R. The pulpal effects of of brushing with a 5 percent potassium nitrate paste used for desensitization. Oral Surg Oral Med Oral Pathol. 1981;51:600–602.
- Olley RC, Moazzez R, Bartlett DW. Effects of dentifrices on subsurface dentin tubule occlusion: an in situ study. Int J Prosthodont. 2015;28:181–187.
- Pearce NX, Addy M, Newcombe R. Dentine hypersensitivity: a clinical trial to compare 2 strontium desensitizing toothpastes with a conventional fluoride toothpaste. J Periodontol. 1994;65:113–119.
- Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21:42–47.
- Panagakos F, Schiff T, Guignon A. Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent. 2009;22:3A–7A.
- Hirsiger C Schmidlin PR, Michaelis M, et al. Efficacy of 8% arginine on dentin hypersensitivity: A multicenter clinical trial in 273 patients over 24 weeks. J Dent. 2019;83:1–6.
- Magno M, Nascimento G, Da Penha N, Pessoa O, Loretto S, Maia L .Difference in effectiveness between strontium acetate and arginine-based toothpastes to relieve dentin hypersensitivity. A systematic review. Am J Dent. 2015;28:40–44.
- Chen C, Parolia A, Pau A. Comparative evaluation of the effectiveness of desensitizing agents in dentinal tubule occlusion using scanning electron microsopy. Aus Dent J. 2015;60:65–72.
- Pradeep A, Sharma A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: a randomized clinical trial. J Periodontol. 2010;81:1167–1173.
- Minkov B, Marmari I, Gedalia I, Garfunkel A. The effectiveness of sodium fluoride treatment with and without iontophoresis on the reduction of hypersensitive dentin. J Periodontol. 1975;46:246–249.
- Miller JT, Shannon IL, Kilgore WG, Brookman JE. Use of water-free stannous fluoride containing gel in the control of dentinal hypersensitivity. J Periodontol. 1969;40:490–491.
- Tal M, Orion M, Gedalia I, Ehrlich J. X–ray diffraction and scanning electron microscope investigations of fluoride-treated dentine in man. Arch Orla Biol. 1976;21:285–290.
- O’Mullane D, Baez R, Jones S, et al. Fluoride and oral health. Community Dental Health. 2016;33:69–99.
- Ritter A, Dias W, Miguez P, Caplan D, Swift E Jr. Treating cervical dentin hypersensitivity with fluoride varnish. J Am Dent Assoc. 2006;137:1013–1020.
- Avad F, Avad N, Vazquez J, Zhang Y, Mateo L, Cummins D. Use of a toothpaste containing 8% arginine and calcium carbonate for immediate and lasting relief of dentin hypersensitivity: a simple and effective in-office procedure. Am J Dent. 2018;31:135–140.
- West N, Seong J, Davies M. Management of dentine hypersensitivity: efficacy of professionally and self-administered agents. J Clin Periodontol. 2015;42(Suppl 16):S256–S302.
- Brannstrom M, Johnson G. Effects of various conditioners and cleansing agents on prepared dentin surfaces. A scanning electron microscopic investigation. J Prosthet Dent.1974;31:422–430.
- Pashley D, Galloway S. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol. 1985;30:731–737.
- Addy M, Dowell P. Dentine hypersensitivity — A review. Clinical and in vitro evaluation of treatments. J Clin Periodontol. 1983;10:351–363.
- Stewardson D, Crisp R, McHugh S, Lendenmann U, Burke F. The effectiveness of Systemp desensitizer in the treatment of dentine hypersensitivity. Prim Dent Care. 2004;11:71–76.
- Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2–hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006;114:354–359.
- Kakaboura A, Rahiotis C, Thamaidis S, Doukoudakis S. Clinical effectiveness of two agents on the treatment of tooth cervical hypersensitivity. Am J Dent. 2005;18:291–295.
- Pashley D. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod. 1986;12:465–474.
- Trushkowsky RD, Oquendo A. Treatment of dentin hypersensitivity. Dent Clin N Am. 2011;55:599–608.
- Chen H, Tang Z, Liu J, et al. Acellular synthesis of a human enamel–like microstructure. Adv Materials. 2006;18:1846–1851.
- Cheng S, Chen H, Liu J, et al. Synthesis of a potentially bioactive hydroxyapatite-nucleating molecule. Calcif Tissue Int. 2006;79:55–61.
- Rezazadeh F, Dehghanian P, Jafarpour D. Laser effects on the prevention and treatment of dentinal hypersensitivity: a systematic review. J Lasers Med Sci. 2019;10:1–11.
- Gordan V, Blaser P, Watson R, et al. A clinical evaluation of a glass ionomer restorative system containing surface prereacted glass ionomer filler: results from a 13-year recall examination. J Am Dent Assoc. 2014;145:1036–1043.
- Leybovich M, Bissada N, Teich S, Demko C, Ricchetti P. Treatment of noncarious cervical lesions by a subepthelial connective tissue graft versus a composite resin restoration. Int J Periodont Res Dent. 2014;34:649–654.
- Tatakis D, Chambrone L, Allen E, et al. Periodontal soft tissue root coverage procedures: A consensus report from the AAP Regeneration Workshop. J Periodontol. 2015;86(Suppl 2):S52–S55.
From Decisions in Dentistry. June 2020;6(6):25–28,31.




