 KIRILLICA/ISTOCK/GETTY IMAGES PLUS
KIRILLICA/ISTOCK/GETTY IMAGES PLUS
Infection Prevention in Endodontic Therapy
Successful outcomes in endodontic therapy depend on sound technique that follows recommended protocols for asepsis and safety.
This course was published in the August 2019 issue and expires August 2022. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the concepts of root canal therapy and instrumentation, and the role asepsis plays in endodontic outcomes.
- Discuss clinical factors that can affect the success of instrument processing in endodontic therapy.
- Describe the demands placed on endodontic files and burs, the impact of cyclical fatigue, and the role of single-use instruments in root canal treatment.
INTRODUCTION
Joseph Lister first used carbolic acid spray in the operating room in 1865 to minimize infections and dramatically improve healing. These basic principles remain relatively unchanged and can be extended to modern endodontic therapy.
In addition to existing pathology and infection, the introduction of new pathogens via improperly sterilized instruments and cross infection carries even greater risk at a time when antibiotic-resistant bacterial strains and so-called “superbugs” can cause rapidly spreading infections with catastrophic results.
After a distinguished career in the U.S. Air Force, Douglas L. Risk, DDS, ABGD, FICD, is a leading proponent of infection control in dentistry, serving on the board of directors of the Organization for Safety, Asepsis and Prevention (OSAP), providing consulting services, and writing and speaking on infection prevention. In this current article, he outlines the pathway to sound clinical outcomes by highlighting effective infection control protocols, as well as pitfalls to avoid, during endodontic treatment.
—Arif Khan, BDS
Senior Manager, Endodontics
Dentsply Sirona, USA
There has been a revolution in endodontic instrumentation and clinical technique over the last 25 years1,2 due largely to the introduction of file systems with new alloys, designs and protocols aimed at increasing the effectiveness and efficiency of root canal shaping and cleaning. The goal of endodontic therapy, however, has remained relatively constant: Eliminate the current or potential microbial infection inside the canal system and prevent reinfection by permanently sealing the canal space, thereby curing or preventing an apical or lateral periodontitis.3 In light of this clinical goal, the importance of following recommended infection prevention protocols becomes clear, as asepsis is crucial to successful endodontic outcomes. This paper will (1) address the risks inherent to endodontic therapy, (2) discuss the benefits of treating endodontic files and other armamentarium as single-use instruments, and (3) outline additional infection prevention techniques to reduce the potential for introducing microorganisms into a canal system during treatment.
With an eye toward effective and aseptic care, this article will also review national regulations and guidelines on disinfection and sterilization of medical instruments, and the challenges dental teams must meet to ensure successful endodontic therapy in an infection-prevention-compliant practice.
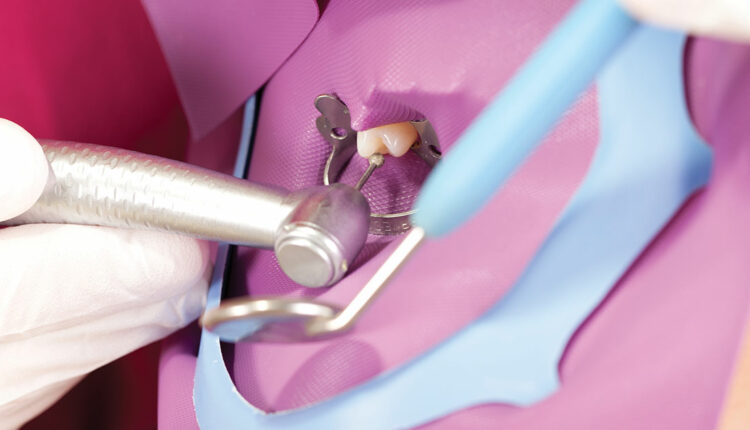
Every endodontic textbook includes an extensive discussion on the complexity of the pulpal space and images of how the space may be convoluted. The operator is challenged to imagine a vision of final obturation that includes all the ribbons and projections of tissue, so these spaces do not become a source of reinfection or reservoir for microbial proliferation. This vision becomes the clinical objective when choosing instruments for therapy.
In its most basic form, the initial phase of endodontic instrumentation involves cutting hard tissue to gain access to the infected or potentially infected soft tissue. Various high-speed and slow-speed burs, hand files and rotary files all have a role in removing enamel and dentin to provide access to potential foci of bacterial infection of the periradicular space. Instruments gaining access through enamel are hard and aggressive. Diamonds and carbide burs gaining access through enamel grind through the material, pulverizing as they generate heat and debris. The debris is partially washed away with irrigation, but the rest is collected on the bur. Once the hard enamel is removed, the softer, more organic dentin is encountered, and more tenacious soft debris is collected as the shaping of the canal access and pulp chamber begins.
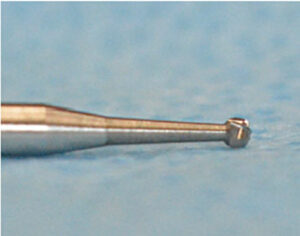
Final access to the canal system is accomplished with slow-speed, finishing and endodontic-specific burs that shape the pulp chamber access, collecting all kinds of organic debris, including blood and neural tissue (Figures 1A–1B and Figures 2A–2B). The instruments used to enter the canal spaces are necessarily smaller and thinner, and the nature of canal filing demands the debris be collected on the file and removed from the canal system coronally — rather than washed laterally, as in the case of enamel cutting. This is a highly contaminated surgical procedure in a very confined space using complex and specialized instruments. Closer to the final shaping and cleaning phase of canal preparation, clinicians are chiefly removing dentin debris mixed with the irrigation solution (Figure 3). Although this debris is less tenaciously attached to the file, if allowed to dry, it becomes more difficult to remove. Irrigation materials also have an effect on the surface of the files and burs, increasing the risk of corrosion (as seen in Figures 1B and 2B).
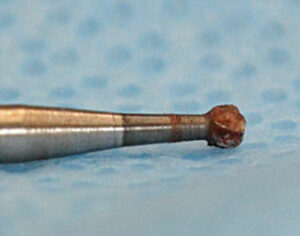
dried over 48 hours, saved specifically for
this photograph and then properly
discarded.
CRITICAL CONSIDERATIONS
Endodontic instruments are designed to contact the contaminated tissue without regard for the immunocompetency of the individual being treated. For this reason, the risk of contamination is significant if nonsterile instruments are used, particularly for immunocompromised patients. As seen in two highly publicized cases in Georgia4 and California,5 systemic infections of pediatric patients have occurred following pulpal therapy; thus, endodontic access and cleaning and shaping of canal systems could lead to the introduction of microorganisms into the systemic circulation and cause significant disease. For this reason, the California Dental Board changed its rules for dentists entering the pulpal tissues and now requires clinicians to use sterile irrigation solutions or disinfecting agents while accessing pulpal tissues in all patients.6 It must be accepted, therefore, that endodontic therapy has the ability to engage the systemic circulation and that instruments used for treatment should be considered critical by the Spaulding criteria.7
The discussion of infection prevention in the endodontic environment is complex and this attempt to address practical issues involving endodontic therapy — including patient safety, disinfection and sterilization, and costs — may elicit contrary points of view. Nonetheless, key questions should be asked in an attempt to critically evaluate the process of instrumenting a canal system in the light of infection prevention and sterilization protocols:
- Does it make sense to reprocess instruments for endodontic therapy?
- What are the risks and benefits of reprocessing? And what are the costs of reprocessing versus the cost of single-use instruments?
- What other protocols exist for infection prevention and safety in root canal therapy?
In 2015, The U.S. Food and Drug Administration (FDA) published a guideline called Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling stating general considerations for reusable medical devices, and what information the directions for use (DFU) should include.8 The FDA acknowledges that medical devices have become more and more complex, and that device reprocessing now also requires advanced knowledge and technology. At the same time, the FDA notes it is more essential than ever for manufacturers to provide simple and clear instructions that facilitate efficient reprocessing of instruments that will be used on subsequent patients. The safety of patients and staff should be the focus of reprocessing directions, and the FDA recommends clarity in manufacturers’ DFU materials. The manufacturer’s indications for use and reprocessing directions should be followed closely so the device is used safely during patient care.

There are many guidelines on sterilization of critical medical devices from the U.S. Centers for Disease Control and Prevention (CDC), Association for Advancement of Medical Instrumentation (AAMI), Healthcare Infection Control Practices Advisory Committee and others advocating that critical items must be thoroughly cleaned before being packaged for sterilization and processed using a validated sterilization method.9 The CDC recommended thorough cleaning of dental instruments prior to sterilization as far back as 1986 in its Recommended Infection Control Practices for Dentistry.10 The AAMI’s ST79:2017 Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities, section 7.6.4.5, recommends that “personnel should visually inspect each item carefully to detect any visible soil.” It provides rationale for this verification, noting, “steam sterilization cannot be assured unless proper cleaning of the device and reduced bioburden and soil was achieved … Cleaning encompasses the removal of organic residues (e.g., blood, tissues, bone fragments, secretions and excretions) and microorganisms from the patient, from handling, and from water exposure during reprocessing. Inspection using enhanced visualization tools, such as lighted magnification … might identify residues not observable by the unaided eye.”11
It is reasonable to conclude this residue list would involve tooth debris from high-speed and slow-speed rotary burs, hand files, and other controlled rotary and oscillating instruments used in endodontic therapy.11 The debris left on burs and files during canal instrumentation, whether loose or “baked on” from frictional heat, might hinder the cleaning and sterilization processes and potentially render these instruments unsterile — even though they went through a steam sterilization cycle. Such instruments must be thoroughly cleaned before being packaged and sterilized.12 It has long been established that residual organic debris and soils have protected microorganisms from the sterilization process. Articles in the scientific literature dating back to 1938 have reported variable contamination residual to sterilization processes. In 1967, Doyle and Ernst13 found that occluded spores of Bacillus subtilis were 900 times more likely to survive sterilization at normal temperatures and time, and that it was only after steam sterilization for 2.5 hours (150 minutes) that total inactivation was attained. The thought that dirt or debris can be sterilized has been proven false for more than 50 years.13
VALIDATED STERILIZATION

and used during treatment; it was saved
specifically for this photograph and then
properly discarded.
As noted, clear and efficient directions on the reprocessing of dental instruments must be followed to provide a validated sterile instrument for patient use. These instructions are often quite involved and provide specific times, temperatures and cleaning protocols. Not all DFUs are the same for instruments of similar design and materials, and DFUs often vary for the different instruments used in the procedure or appointment. Dental teams should review DFUs prior to purchasing instruments or systems — especially complex technologies, such as obturation systems containing multiple parts, each with its own disinfection and sterilization instructions. The goal is to seamlessly incorporate the reprocessing protocol into existing routines.
In other words, it would not be prudent to disrupt an instrument processing center’s workflow for a single instrument unless that instrument is so critical that disruption is justified. For example, if routine instrument cleaning involves a 15-minute ultrasonic cleaning cycle and one of the instruments requires a 30-minute cycle, this would be disruptive to the routine workflow. When an equivalent instrument is available (of identical function and quality) that would not disrupt workflows, the team would realize efficiencies in processing costs and staff time.
In response to the key questions to consider when evaluating infection prevention and sterilization protocols for endodontic therapy, consider the following discussion.
Does it make sense to reprocess instruments for endodontic therapy?
A search of DFUs for reprocessing various nickel-titanium endodontic files reveals wide discrepancies, as some instructions are quite specific and detailed, while others are either vague or not specified. In terms of single-patient instruments, some products must be sterilized before use, but single-patient files are also available presterilized. From an infection control perspective, the latter might be considered an ideal instrument inasmuch that, if properly used, there is no chance for any user-induced contamination for any patient treated. It is acknowledged that not all single-patient files are supplied this way, and any instruments that must be sterilized prior to use would add to the cost due to staff time needed for processing. Additionally, if the processing instructions are complex and time intensive, the cost is greater than if DFUs match the office’s existing protocol.
Instrument processing is a critical step in any treatment setting and must be carried out with meticulous precision — every time and with each instrument — to ensure patient and practitioner safety. From a practical standpoint, it would be optimal to choose a file system that provides the best outcomes in the hands of the operator and is either provided sterile or reprocessed like other instruments in the practice. The bottom line is that it makes sense to reprocess instruments only if it can be done meticulously, consistently, precisely and cost-effectively. If not, the practice should consider sterile single-use files that are discarded immediately after the appointment.
This brings up a key point: Offices should never reprocess and reuse single-use endodontic files on subsequent patients. Beyond the possibility of inadequate sterilization due to the potential for residual or baked-on debris, there are concerns over cyclical fatigue that can lead to file separation. Multiple-patient use of a single-use instrument increases the risk of breakage, iatrogenic complication and loss within the canal.
What are the risks and benefits of reprocessing? And what are the costs of reprocessing versus the cost of single-use instruments?
The risks of reprocessing endodontic files, diamond burs, carbide burs and other devices that tend to collect debris under frictional heat or are otherwise difficult to clean are several. As noted, Doyle and Ernst13 found organic debris shielded spores from steam sterilization for up to 150 minutes at 121°C. If files, burs or other instruments are not absolutely clean prior to sterilization, sterility cannot be assured. Endodontic instruments may have the advantage of operating in an environment in which disinfectants are used as irrigants, but that does not negate the need to follow the accepted aseptic principle of using only sterile instruments.
If these instruments were manufactured as single-patient items and sterilized before shipment, a critical step would be eliminated in the assurance of patient safety. The initial cost of sterilization at the practice level would be eliminated, and the products could be used immediately. The benefits to the office would be significant: Not having to worry about whether the instruments were processed correctly, and never having to worry that a bur may have gotten into the drain mechanism of an automated washer (a $60,000 device vulnerable to small instruments causing catastrophic malfunction).
An FDA approved and validated sterilization process — meticulously followed prior to use of the instrument — is a significant contributor to the provision of safe and effective endodontic care. Yet dental teams also have the option of using presterilized, single-patient instruments. In this scenario, many clinicians might choose a single-use instrument that was shipped sterile, as opposed to one that needed to be processed per the manufacturer’s instructions prior to use. While reprocessing multiple-use instruments is necessary for safe care, the staff time needed for cleaning and sterilization adds to the cost of the procedure. If a sterile alternative were available, it would be a valuable clinical option. An additional consideration is that risks of reprocessing are commensurate with the diversity of dental settings and personnel who work in instrument processing centers.
What other protocols exist for infection prevention and safety in root canal therapy?
Successful sterilization of instruments is a key tenet of safe care. As previously discussed, endodontic instruments have access to the bloodstream and systemic circulation, and should be considered critical instruments per Spaulding’s classification. Beyond reprocessing, additional protocols contribute to infection prevention in endodontic therapy. The most visible protocol advocating patient safety is the dental dam. By having a well-sealed barrier in place, use of dams eliminates many of the opportunities for contamination and untoward events. Root canal treatment is one of the safest procedures from an operator control perspective thanks to Dr. Sanford Barnum’s creation in 1864.14 The dental dam is a single-use device with a good history of minimal adverse reactions, and nonlatex materials have made the device even safer.
Another infection prevention protocol (or set of protocols) involves irrigation. Disinfection, elimination of tissue and organic debris, removal of the smear layer, and softening of dentin are among the tasks asked of irrigants. Since no single product achieves all the desired functions necessary for successful endodontic therapy, a combination protocol is most often followed. Sodium hypochlorite, which remains one of dentistry’s best broad-spectrum antimicrobials, is a common disinfectant in root canal treatment, and, when used in judicious combination with other chemicals, can effectively achieve tissue dissolution, as well as disinfection.15
All solutions should be prepared fresh for each treatment; sodium hypochlorite, for example, can degrade based on factors that include temperature and exposure to oxygen and light.16 In addition, organic solvents will degrade syringe material in an unpredictable way, rendering the solution contaminated at the point of use in very short time.17 For these reasons, solutions should be prepared immediately before use in a manner consistent with the practice’s hazard communication plan required by the Occupational Safety and Health Administration.18
FILE SEPARATION
One of the most vexing events in root canal treatment is file separation. The endodontic literature addresses the various factors that influence file separation, and though nickel-titanium offers increased flexibility and fracture resistance compared to stainless steel files, increased operator stress still puts these instruments at risk for breakage. File curvature and abruptness or radii of the curvature are key components in instrument fatigue.19 Therefore, another justification for single use of endodontic files is the metal fatigue they undergo during treatment of a single tooth, especially multirooted teeth of unknown curvature. Instrumenting the third, fifth, seventh or ninth canal easily could cause enough stress to lead to fracture.
Technique also plays a role in metal fatigue and the number of cycles that lead to failure. Some files have been shown to be more fracture resistant when used with specific techniques of high torque and manual preflaring.20 In root canal therapy, the techniques needed to produce a clean canal may fatigue a file prematurely or perhaps in unpredictable ways. As noted earlier, clinicians should never sterilize and reuse instruments intended as single-patient-use files due to the increased risk of breakage and iatrogenic complication.
PRACTICAL CONSIDERATIONS
In dental practice, non-dentist team members perform most compliance-related tasks, and the number of jobs these staff members must handle is almost insurmountable. There is always a prioritized list of things to do. For example, one of the most critical responsibilities is to be on time: Seat the patient on time, finish on time, and do not make anyone wait. While widely acknowledged as an important component of safe care, infection prevention may not always be ranked as a top priority at all times in every office. Dental manufacturers can make a significant contribution to contamination-free endodontic treatment by offering aseptically packaged, single-patient instruments that are presterilized and ready for immediate use.
Because clinicians are responsible for patient safety, dental teams may find it most efficient to open a sterile pack chairside for the doctor and place it in the sharps container when treatment is finished. Ultimately, when it comes to endodontic asepsis, instruments should integrate into clinical practice, not the other way around. Thus, the profession may gravitate toward the sterile packaging of single-use burs, endodontic files and similar instruments — an approach that will ensure sharp instruments and minimize risks associated with reprocessing, cyclical fatigue and breakage.
CONCLUSION
Dental patients have the right to expect quality care and safe treatment, and protocols with regard to individualized treatment plans and infection control should be in place to ensure successful endodontic therapy. Without raising overall costs or overwhelming the staff, oral health professionals should take the initiative on patient safety in the dental chair. A trend toward sterile product delivery and single-use instruments would make the profession’s future brighter than ever.
REFERENCES
- Kuzekanani M. Nickel-titanium rotary instruments: Development of the single-file systems. J Int Soc Prev Community Dent. 2018;8:386–390.
- Gavini G, Santos MD, Caldeira CL, Machado ME, Freire LG, Iglecias EF, Peters OA, Candeiro GT. Nickel-titanium instruments in endodontics: a concise review of the state of the art. Braz Oral Res. 2018;32(Suppl 1):e67.
- Peters OA, Pewters CI, Basrani B. Cleaning and shaping the root canal system. In: Hargreaves KM, Berman LH, Rothstein I, eds. Cohen’s Pathways of the Pulp. 11th ed. St Louis, Mo: Elsevier; 2016.
- Peralta G. Notes from the field: Mycobacterium abscessus infections among patients of a pediatric dentistry practice — Georgia, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:355–356.
- Ross E. Infection Outbreak Shines Light on Water Risks at Dentists Offices. Available at: https://www.npr.org/sections/health-shots/2016/09/30/495802487/infection-outbreak-shines-light-on-water-risks-at-dentists-offices. Accessed June 25, 2019.
- Dental Practice Act. California SB 1491, Chapter 703. Available at: http://leginfo.legislature.ca.gov/faces/billPdf.xhtml?bill_id=201720180SB1491&version=20170SB149194CHP. Accessed June 25, 2019.
- Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Guidelines for infection control in dental health-care settings — 2003. MMWR Recomm Rep. 2003;52(RR-17):1–61.
- U.S. Food and Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling — Guidance for Industry and Food and Drug Administration Staff. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reprocessing-medical-devices-health-care-settings-validation-methods-and-labeling. Accessed June 25, 2019.
- Rutala WA, Weber DJ. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/. Accessed June 25, 2019.
- U.S. Centers for Disease Control and Prevention. Recommended Infection-Control Practices for Dentistry. Available at: https://www.cdc.gov/mmwr/PDF/rr/rr4208.pdf. Accessed June 25, 2019.
- American National Standards Institute/Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST79:2017. Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Available at: http://my.aami.org/aamiresources/previewfiles/1709_ST79Preview.pdf. Accessed June 25, 2019.
- Parashos P, Linsuwanont P, Messer HH. A cleaning procotol for rotary nickel-titanium endodontic instruments. Aust Dent J. 2004:49:20–27.
- Doyle JE, Ernst RR. Resistance of Bacillus subtilis var. niger spores occluded in water-insoluble crystals to three sterilization agents. Appl Microbiol. 1967:15:726–730.
- Ahmad IA. Rubber dam usage for endodontic treatment: a review. Int Endod J. 2009;42:963–972.
- Zehnder M. Root canal irrigants. J Endod. 2006;32:389–398.
- Torabinejad, M. Root canal irrigants and disinfectants. Available at: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/rootcanalirrigantsdisinfectants.pdf. Accessed June 25, 2019.
- Mykin Inc. Rubber Chemical Resistance Chart. Available at: http://mykin.com/rubber-chemical-resistance-chart. Accessed June 25, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration Standard Number 1910.1200. Hazard Communication, Toxic and Hazardous Substances. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10099&p_table=STANDARDS. Accessed June 25, 2019.
- Pruett JP, Clement DJ, Carnes DL Jr. Cyclic fatigue testing of nickel-titanium endodontic instruments. J Endod. 1997:23;77–85.
- Berutti E, Negro AR, Lendini M, Pasqualini D. Influence of manual preflaring and torque on the failure rate of ProTaper rotary instruments. J Endod. 2004:30:228–230.5
From Decisions in Dentistry. July/August 2019;5(7):29—34




