The Expanding Role of Photobiomodulation
Once used mainly for cutting, lasers are now transforming dental care through photobiomodulation.
Lasers have traditionally been used for excisional procedures, but over the past two decades, important nonsurgical applications have emerged. Novel modifications in laser technology now allow their use to accelerate healing, reduce inflammation, and diminish pain — all noninvasively.
This approach is termed photobiomodulation or photobiomodulation therapy and is defined as the targeted application of light for therapeutic purposes at a level below that associated with cutting of tissue or damage to structural proteins. The concept was introduced in 1971 and has been thoroughly researched since that time.1,2
 Photobiomodulation delivers photons of light to the affected area and can be generated by a variety of lasers. The lasers that can develop these photons can be differentiated by the wavelength of light generated and the depth of penetration of the energy produced. Early generations of these devices could only deliver power sufficient to penetrate superficial tissues. This low-powered process is most frequently termed low-level laser therapy (LLLT). These lasers, by definition, produce 0.5 watts or less of power, which limits the depth of tissue penetration. The wavelengths produced by these devices range from 390 to 600 nm. More powerful lasers can deliver light energy to deeper tissues, a process termed high-intensity laser therapy (HILT), and generate wavelengths ranging from 810 to 1,064 nm. Both LLLT and HILT are noninvasive, with minimal negative side effects and both positively affect numerous cellular processes.
Photobiomodulation delivers photons of light to the affected area and can be generated by a variety of lasers. The lasers that can develop these photons can be differentiated by the wavelength of light generated and the depth of penetration of the energy produced. Early generations of these devices could only deliver power sufficient to penetrate superficial tissues. This low-powered process is most frequently termed low-level laser therapy (LLLT). These lasers, by definition, produce 0.5 watts or less of power, which limits the depth of tissue penetration. The wavelengths produced by these devices range from 390 to 600 nm. More powerful lasers can deliver light energy to deeper tissues, a process termed high-intensity laser therapy (HILT), and generate wavelengths ranging from 810 to 1,064 nm. Both LLLT and HILT are noninvasive, with minimal negative side effects and both positively affect numerous cellular processes.
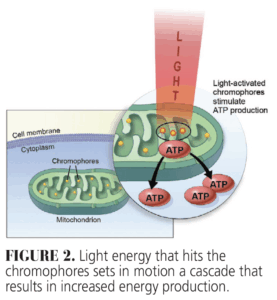
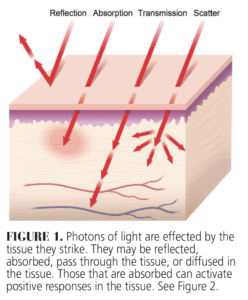
Of greatest clinical significance is the energy absorbed by molecules in the tissue called chromophores. This because it leads to the production of energy, which results in many of the positive responses seen following photobiomodulation. Some energy may also be transmitted through the tissue, while other photons can be scattered and yet others are reflected thus diminishing the positive effects of therapy (Figure 1). When energy is absorbed, it produces photochemical effects, including increasing cellular adenosine triphosphate (ATP) production by targeting mitochondrial chromophores (Figure 2).3
HILT is able to produce high peak power (in the kilowatt range) and short pulses with a low duty cycle (0.1% to 0.01%), ensuring sufficient cooling of tissues between emissions. Unlike high-power lasers (HPL) that operate with higher duty cycles (10% to 100%) and lower peak power, HILT uniquely induces the photomechanical effects through the generation of photoacoustic waves.
These waves directly impact the extracellular matrix and cytoskeleton, triggering cascades of biological responses that promote tissue regeneration and repair.4 Additionally, HILT can be adjusted to longer pulses for controlled photothermal effects,5,6 which propagate thermal energy through tissue to activate cellular heat shock proteins (HSPs), such as HSP47 and HSP72. These proteins play critical roles in tissue remodeling, particularly in fibrotic conditions.6
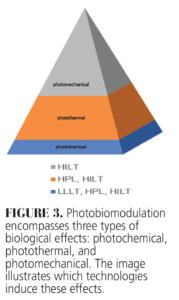
Photobiomodulation can induce photochemical effects across all laser types. Both HPL and HILT are capable of producing combined photochemical and photothermal effects. However, HILT induces all three effects: photochemical, photothermal, and photomechanical (Figure 3). This unique capability makes HILT suitable for applications requiring deep tissue penetration and more comprehensive biological modulation. To achieve these tailored therapeutic outcomes, careful consideration of laser parameters is essential.
 The photothermal effect results in vasodilation. Clinically, HILT has been shown to stimulate blood flow through angiogenesis and extracellular matrix synthesis that can lead to more rapid healing times for oral wounds.7Another important part of stimulating the healing process is the effect of photobiomodulation on susceptible stem cells and progenitor cells as well as its potential to enhance cell differentiation, all of which, in turn, improve the rate of healing.8 Multiple studies have reported that stem cell proliferation is improved by photobiomodulation, including the production of gingival fibroblasts. Its effect on dental pulp stem cells extracted from permanent teeth, and exfoliated deciduous teeth has also been studied.9
The photothermal effect results in vasodilation. Clinically, HILT has been shown to stimulate blood flow through angiogenesis and extracellular matrix synthesis that can lead to more rapid healing times for oral wounds.7Another important part of stimulating the healing process is the effect of photobiomodulation on susceptible stem cells and progenitor cells as well as its potential to enhance cell differentiation, all of which, in turn, improve the rate of healing.8 Multiple studies have reported that stem cell proliferation is improved by photobiomodulation, including the production of gingival fibroblasts. Its effect on dental pulp stem cells extracted from permanent teeth, and exfoliated deciduous teeth has also been studied.9
Photobiomodulation can also be used to stimulate the immune response. Near infrared wavelengths (HILT) increase the production of nitric oxide, a compound that aids in healing cells and increases blood flow. Nitric oxide regulates the activation, proliferation, and differentiation of immune cells. White blood cells release nitric oxide when detecting a threat, so increasing nitric oxide levels that can stimulate the immune system, regulate blood pressure, and reduce inflammation.10
Dental Applications
Photobiomodulation has been used in dentistry for decades. Until recently, the majority of applications employed LLLT. Early studies on the use of LLLT to treat temporomandibular joint problems had mixed results but subsequent research using HILT frequently reduced pain, increased range of motion, and decreased disability compared to LLLT.11
Photobiomodulation can also reduce dental pain, be used to induce dental anesthesia,12 reduce dental hypersensitivity,13 and diminish pain following orthodontic band placement.14 A meta-analysis of pain relief using photobiomodulation demonstrated that laser phototherapy was very efficacious in relieving pain.15
The effect of photobiomodulation on orthodontic tooth movement has been studied extensively. One review of wavelengths between 780 and 830 nm showed an increase in the speed of tooth movement vs controls by 24%.16 Another group that received HILT had accelerated tooth movement during molar uprighting compared to controls.17 A recent review concluded that photobiomodulation can effectively reduce orthodontic treatment time and produce anesthesia and anti-inflammatory effects. A concern of the reviewers was a lack of consistency among photobiomodulation protocols.18
Of clinical significance is this therapy’s effect on the healing of bone. Photobiomodulation has been shown to accelerate healing in extraction sockets in both animal and human models.19-21 It has also been shown to speed bone healing around dental implants. One study of bone deposition around dental implants in rabbit tibia using HILT concluded that laser photobiomodulation accelerated bone healing around these devices compared to controls.22 In one animal study, photobiomodulation was found to accelerate bone healing by stimulating blood flow, activating osteoblasts, and diminished osteoclastic activity when used in extraction sites augmented with hydroxyapatite biomaterial.23 One study of dental implants placed into rat femurs and treated with either 660 nm or 808 nm lasers, found increased bone tissue matrix production compared to controls.24
A number of studies have looked at the effect of photobiomodulation therapy on treating erosive lichen planus. The majority indicate that higher intensity levels reduce or remove the necessity for more aggressive forms of therapy, making laser therapy the safer alternative. Specifically, HILT has been found to be superior to surgical removal of these lesions25 and has also been shown to reduce pain associated with these lesions.26 Using HILT often eliminates the need for corticosteroid therapy, thus reducing the possible systemic problems associated with this form of therapy.
Conclusion
Photobiomodulation is safe and effective. Applications using LLLT are best reserved for superficial tissues such as gingiva. It seems reasonable that the use of longer wavelengths produced by HILT will have a more profound effect and thus work better for most hard tissue applications. One drawback to the use of photobiomodulation is the lack of specific wavelengths and settings for specific therapeutic applications. Unfortunately, the studies on this topic are heterogeneous, and further study is needed to gain more specificity. Research is ongoing to specifically find the type of laser and the settings for that laser for each individual dental application. It is undoubtedly true that this technology will advance with our knowledge of more directed applications and thus be beneficial for many of our patients.
Acknowledgment
The authors would like to thank Damiano Fortuna, DVM, and Kathryn Mootz, MBA, for their help with this manuscript.
References
- Mester E, Ludany G, Selyei M, Szende B, Total GJ. The stimulating effect of low power laser rays on biological systems. Laser Rev. 1968;3:12-14.
- Rodriguez Salazar DY, Malaga Rivera JA, Laynes Effio JE, Valencia-Arias A. A systematic review of trends in photobiomodulation in dentistry between 2018 and 2022: advances and investigative agenda. F1000Res. 2023;12:1415.
- Zati A, Valent A. Physical therapy: new technologies in rehabilitation medicine (translated to English). Edizioni Minerva Medica. 2006;2006:162-185.
- Fortuna D, Masotti L. The HILT domain by the pulse intensity fluence (PIF) formula. Energy for Health. 2010;5(5):12-19.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5:58-62.
- Cronshaw M, Parker S, Grootveld M, Lynch E. Photothermal effects of high-energy photobiomodulation therapies: an in vitro investigation. Biomedicines. 2023;11:1634.
- da Silva Neves FL, Silveira CA, Dias SB, et al. Comparison of two power densities on the healing of palatal wounds after connective tissue graft removal: randomized clinical trial. Lasers Med Sci. 2016;31:1371-1378.
- de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:2561201
- Fernandes AP, Junqueira Mde A, Marques NC, et al. Effects of low-level laser therapy on stem cells from human exfoliated deciduous teeth. J Appl Oral Sci. Jul-Aug 2016;24:332-337.
- Barolet AC, Litvinov IV, Barolet D. Light-induced nitric oxide release in the skin beyond UVA and blue light: red & near-infrared wavelengths. Nitric Oxide. 2021;117:16-25.
- Ekici O, Dundar U, Buyukbosna M. Effectiveness of high-intensity laser therapy in patients with myogenic temporomandibular joint disorder: A double-blind, placebo-controlled study. J Stomatol Oral Maxillofac Surg. 2022;123:e90-e96.
- Poli R, Parker S. Achieving dental analgesia with the erbium chromium yttrium scandium gallium garnet laser (2780 nm): A Protocol for Painless Conservative Treatment. Photomed Laser Surg. 2015;33:364-371.
- Cattoni F, Ferrante L, Mandile S, Tete G, Polizzi EM, Gastaldi G. Comparison of lasers and desensitizing agents in dentinal hypersensitivity therapy. Dent J (Basel). 2023;11:11030063.
- Sfondrini MF, Vitale M, Pinheiro ALB, et al. Photobiomodulation and pain reduction in patients requiring orthodontic band application: randomized clinical trial. Biomed Res Int. 2020;2020:7460938.
- Kate RJ, Rubatt S, Enwemeka CS, Huddleston WE. Optimal laser phototherapy parameters for pain relief. Photomed Laser Surg. 2018;36:354-362.
- Dominguez Camacho A, Montoya Guzman D, Velasquez Cujar SA. Effective wavelength range in photobiomodulation for tooth movement acceleration in orthodontics: a systematic review. Photobiomodul Photomed Laser Surg. 2020;38:581-590.
- Murakami-Malaquias-Silva F, Perim Rosa E, Malavazzi TCS, et al. Photobiomodulation increases uprighting tooth movement and modulates IL-1beta expression during orthodontically bone remodeling. J Biophotonics. 2023;16:e202300013.
- Goncalves A, Monteiro F, Brantuas S, et al. Clinical and preclinical evidence on the bioeffects and movement-related implications of photobiomodulation in the orthodontic tooth movement: A systematic review. Orthod Craniofac Res. 2025;28:12-53.
- Daigo Y, Daigo E, Hasegawa A, Fukuoka H, Ishikawa M, Takahashi K. Utility of High-intensity laser therapy combined with photobiomodulation therapy for socket preservation after tooth extraction. Photobiomodul Photomed Laser Surg. 2020;38:75-83.
- Daigo Y, Daigo E, Fukuoka H, Fukuoka N, Ishikawa M, Takahashi K. Wound healing and cell dynamics including mesenchymal and dental pulp stem cells induced by photobiomodulation therapy: an example of socket-preserving effects after tooth extraction in rats and a literature review. Int J Mol Sci. 2020;21:21186850.
- Amaroli A, Colombo E, Zekiy A, Aicardi S, Benedicenti S, De Angelis N. Interaction between laser light and osteoblasts: photobiomodulation as a trend in the management of socket bone preservation-a review. Biology (Basel). 2020;9:9110409.
- Lopes CB, Pinheiro AL, Sathaiah S, Da Silva NS, Salgado MA. Infrared laser photobiomodulation (lambda 830 nm) on bone tissue around dental implants: a Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg. 2007;25:96-101.
- Dalapria V, Marcos RL, Bussadori SK, et al. LED photobiomodulation therapy combined with biomaterial as a scaffold promotes better bone quality in the dental alveolus in an experimental extraction model. Lasers Med Sci. 2022;37:1583-1592.
- da Fonseca G, Cavalcanti M, de Souza Maior JD, et al. Laser-photobiomodulation on titanium implant bone healing in rat model: comparison between 660- and 808-nm wavelength. Lasers Med Sci. 2022;37:2179-2184.
- Tarasenko S, Stepanov M, Morozova E, Unkovskiy A. High-level laser therapy versus scalpel surgery in the treatment of oral lichen planus: a randomized control trial. Clin Oral Investig. 2021;25:5649-5660.
- Khater MM, Khattab FM. Efficacy of 1064 Q switched Nd:YAG laser in the treatment of oral lichen planus. J Dermatolog Treat. 2020;31:655-659.
From Decisions in Dentistry. October/November 2025;11(5):14-15.