Synchronous Trauma-Induced and Spontaneous Osteonecrosis of the Jaws
Newer targeted therapies are being linked with medication-related osteonecrosis of the jaw, which underscores the need to stay abreast of its risk factors and etiologies.
This course was published in the February 2021 issue and expires February 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe risk factors for medication-related osteonecrosis of the jaw (MRONJ), including gender prevalence and anatomical sites most commonly affected.
- Explain diagnostic considerations and staging for MRONJ lesions.
- Discuss screening, prevention and management of MRONJ in patients undergoing targeted cancer therapies.
Molecular targeted therapy is being increasingly used for cancer treatment. These drugs focus on specific cancer related genes/proteins or modulate the tumor microenvironment to control growth and survival of cancer cells.1 These therapeutic agents include angiogenesis inhibitors (e.g., bevacizumab and sunitinib); hormone therapies (e.g., anastrozole); signal transduction inhibitors (e.g., imatinib); apoptosis inducers (e.g., venetoclax); monoclonal antibodies that deliver toxic molecules (e.g., inotuzumab ozogamicin); immunotherapy (e.g., pembrolizumab); and bone targeted therapy (e.g., bisphosphonates and denosumab).1 Bone targeted therapies are commonly used for management of multiple myeloma, metastatic cancers to bone and osteoporosis.2 While bisphosphonates, such as pamidronate, zoledronate and alendronate, are known to be associated with medication-related osteonecrosis of the jaw (MRONJ), newer targeted cancer therapies are being implicated in MRONJ, thereby necessitating dental providers to frequently update their knowledge base of this topic.3 For instance, denosumab, bevacizumab, everolimus, sorafenib and sunitinib are other targeted therapies associated with MRONJ.
Most cases of MRONJ are attributed to trauma resulting from dentoalveolar surgery,4–6 however, a small subset of patients may develop this condition spontaneously.7 This report discusses risk factors, diagnosis, management and prevention strategies for MRONJ. It also presents a case of a synchronoustrauma-induced MRONJ lesion in the maxilla and a spontaneous lesion in the mandible associated with denosumab therapy.
Definition and Risk Factors for MRONJ: In 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) updated the definition of MRONJ, which is now defined as exposed bone or bone that is palpable by probing an intra- or extraoral fistula that is persistent for more than eight weeks in the maxillofacial region. The patient must have current/previous treatment with antiresorptive or antiangiogenic drugs, with no history of radiation therapy or metastatic disease to the jaws.2
Local risk factors, such as dental extractions, are a common predisposing event in 66% to 77% of MRONJ lesions.4–6 Concomitant periodontal and periapical disease have been noted in more than 50% of cases, and ill-fitting prostheses in 48% to 77% cases.4–6 Additionally, MRONJ lesions are more likely to develop in the mandible (73%) than the maxilla (22.5%) or both jaws (4.5%).8
Targeted therapy associated risk factors include the duration, dose, dosing interval and oral/intravenous administration. For instance, oncology patients with bone metastases receive denosumab (Xgeva) 120 mg every four weeks or zoledronate (Zometa) 4 mg every three weeks, whereas an osteoporosis patient will likely receive denosumab (Prolia) 60 mg every six months or zoledronate (Reclast) 5 mg/year. The risk of MRONJ is almost 100 times higher in oncology patients who receive higher dosage of antiresorptive drugs via intravenous infusions compared to osteoporotic patients who receive much lower doses via an oral route.2 Risk of denosumab-related MRONJ ranges from 70 to 90 cases per 10,000 in oncology patients9 and approximately four cases per 10,000 in osteoporosis patients.10
Demographic and systemic risk factors include comorbidities, such as diabetes and anemia (hemoglobin < 10 dL), corticosteroid therapy, and concurrent anti-angiogenic therapy.8 Sequential anti-resorptive therapy, such as bisphosphonates followed by denosumab or pamidronate followed by zoledronate, may lead to increased risk of MRONJ.7 Certain cancer types, such as multiple myeloma, breast, prostate, colorectal and renal cell carcinomas, are associated with a higher prevalence of MRONJ,11 likely due to a higher frequency of osseous involvement. Similarly, a higher prevalence of MRONJ in females may be an expression of a high incidence of osteoporosis and breast cancer compared to other conditions that require antiresorptive therapy.2
CASE REPORT
A 66-year-old male presented to the oral oncology and maxillofacial prosthodontics service at the University of Texas MD Anderson Cancer Center (MDACC) in July 2018 with a chief complaint of pain in the maxillary right posterior quadrant. He was otherwise asymptomatic.
The patient had an oncologic history of a T3N1M0 intermediate-grade pancreatic endocrine tumor and lymph node metastasis in 2011. He underwent chemotherapy with FOLFIRINOX (folinic acid, fluorouracil, irinotecan and oxaliplatin) in January 2012, and FAS (5-fluorouracil, doxorubicin and streptozocin) in March 2012. He was also diagnosed with non-small cell bronchoalveolar lung carcinoma in June 2012, for which he received stereotactic radiation, 50 Gy/4 fractions. Due to progression of the pancreatic disease, he underwent distal pancreatectomy with en bloc splenectomy in October 2012. He was closely monitored for several years until 2016, when he was found to have developed further metastasis to the liver and bone. He started somatostatin and subcutaneous denosumab 120 mg every four weeks from January 2017 to March 2018. Other relevant medical history included uncontrolled diabetes mellitus that developed post-pancreatectomy, macrocytic anemia, gastroesophageal reflux disease and hyperlipidemia. The patient was a heavy tobacco smoker with an 80 pack-year history.
The patient revealed he did not have any oral evaluation or counseling prior to denosumab therapy. In December 2017, he underwent extraction of fractured tooth #5 at a local dental office while continuing denosumab. The extraction resulted in a nonhealing socket with purulent discharge. Denosumab was stopped after signs of MRONJ were noted. He consulted another local dentist in May 2018 and was placed on clindamycin and lidocaine oral rinses.
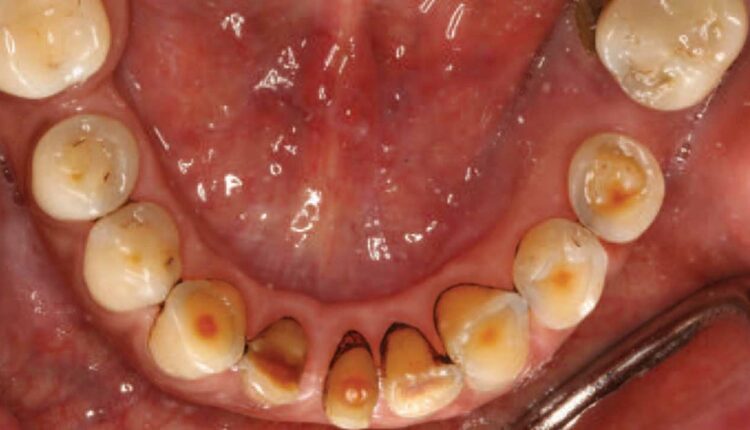
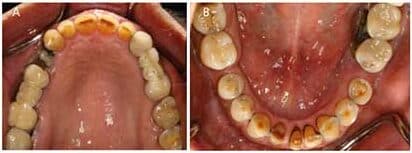
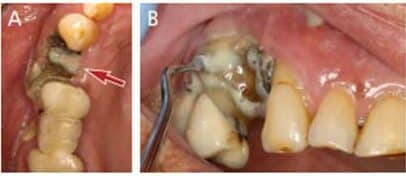
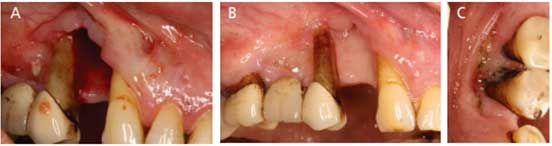
At initial presentation to the MDACC oncology department, the patient had poor oral hygiene, generalized moderate periodontal disease, and bony exposure in the right maxillary and left mandibular quadrants (Figures 1A and 1B). The right maxillary exposure extended from tooth #3 to distal surface of #6, with a mobile exposed bony segment around extraction site #5 (Figures 2A and 2B). Left mandibular bony exposure extended along the lingual surfaces of teeth #17 and 18, with minor mobility of the necrosed lingual cortices (Figures 3A and 3B). Exudate was noted in both maxillary and mandibular lesions.
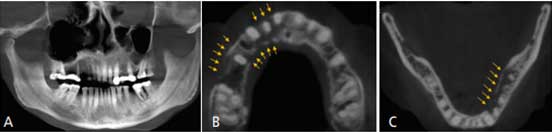
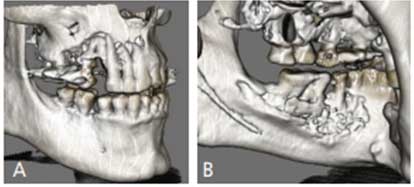
Radiographic evaluation with cone beam computed tomography (CBCT) revealed a poorly defined radiolucency in the maxilla extending from tooth #2 to 8, with complete loss of buccal bone around teeth #4 and 6 (Figure 4A). Axial slices showed the extent of cortical plate destruction and bone involvement in the maxilla, which included buccal cortex from tooth #2 to 8, and palatal cortex from #4 to 8 (Figure 4B). Similarly, complete lingual cortical plate destruction (erosion) was noted from distal of tooth #17 to 22 (Figure 4C) in the mandible. Occluso-cervically, the lesion involved the entire length of the #6, 7, 8, 17 and 18 roots without extension to the sinus/nasal cavity or the inferior border of the mandible (Figures 5A and 5B). Radiographic changes revealed greater bony involvement than the clinical presentation.
Management: At initial presentation, exposed bony fragments were noted to have minimal mobility, which did not allow for atraumatic debridement of the lesions. The patient was prescribed systemic antibiotic therapy, amoxicillin-clavulanate 875/125 twice daily for 10 days. Additionally, he was placed on 0.12% alcohol-free chlorhexidine gluconate twice daily, and 2% lidocaine rinse as needed for pain control. He was referred for smoking cessation therapy and was placed on a monthly recall due to the extent of the disease.
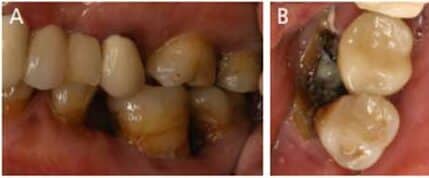
One month later, sequestrectomy was performed in the maxilla based on the mobility of necrosed bone. Soft tissue epithelization was noted underneath the removed bony segment (Figure 6A). The sample was submitted for pathology and the report confirmed devitalized bone and acute and chronic inflammation consistent with osteonecrosis; however, it was negative for tumor. The patient was advised to continue chlorhexidine rinses and was initiated on salt and baking soda rinses five times daily. Smoking cessation was further reiterated, as he continued to smoke.
At his third visit, good healing was evident in the maxilla (Figure 6B), while progression of the mandibular lesion was noted. Sequestrectomy was performed in the mandible and the patient was advised to continue mouthrinses. At his fourth visit, improved healing was noted in the mandible (Figure 6C); however, progression of exposed bone in the maxillary canine area with presence of exudate was evident. Minimal sequestrectomy was performed and the patient was again placed on amoxicillin-clavulanate 875/125 twice daily for 10 days, with continuation of mouthrinses. Unfortunately, further development of osteonecrosis could not be evaluated, as the patient succumbed from a gastrointestinal complication shortly after.
DISCUSSION
As evidenced by this case report, MRONJ is an uncommon, but debilitating, long-lasting and (in some cases) slowly developing disease. Although this patient presented with clinical bony exposure around three teeth in the maxilla and two teeth in the mandible, the true extent of the lesion involved the entire right maxillary quadrant and at least the entire left mandibular posterior sextant. Inevitably, this patient would have eventually lost all supporting structures affected by MRONJ. As a majority of MRONJ cases are precipitated by dentoalveolar surgeries, these lesions can be largely prevented with thorough risk evaluation.
As noted, the patient had a dental extraction in the right maxillary quadrant that resulted in osteonecrosis. Even though the lesion noted in the left mandible presented as a spontaneous lesion with no history of trauma, the patient had several risk factors, including periodontitis, diabetes, macrocytic anemia, smoking, and denosumab therapy with concurrent antiangiogenic chemotherapy, which likely led to osteonecrosis.
Management of MRONJ: The AAOMS recommends treatment of MRONJ based on staging of the lesion(s):2
Stage 0: No clinical evidence of necrosis, but nonspecific clinical findings, radiographic changes and symptoms require systemic management with antibiotics and pain medication.
Stage 1: Presence of asymptomatic exposed/probable necrotic bone without evidence of infection requires an antibacterial mouthrinse with a quarterly clinical follow-up, patient education, and review of indications for continuing antiresorptive therapy.
Stage 2: Exposed, necrotic bone or probable fistulas associated with infection, as evidenced by pain and erythema with or without purulence; commonly requires systemic antibiotics, oral antibacterial mouthrinse and pain control. Controlled debridement of bony sequestrum is indicated to relieve soft tissue irritation and allow better infection control. Debridement should be done without exposing uninvolved bone to avoid further trauma.
Stage 3: Advanced disease includes exposed and necrotic bone extending beyond the alveolar bone (i.e., to inferior border or ramus of the mandible, maxillary sinus or zygoma of the maxilla), or resulting in pathologic fractures, extraoral fistula, or oro-antral or oro-nasal communications. Such cases often require surgical debridement or resection for palliation of infection and pain, in addition to local and systemic antibiotics and pain medications.
Based on the AAOMS criteria, this case was determined to be Stage 2 osteonecrosis. As evidenced by this patient, the initial clinical presentation of the disease is often miniscule when compared to its actual pathological extent, which may or may not appear as early radiographic changes. Therefore, it is crucial that upon initial evaluation patients with MRONJ are counselled about the potential of disease progression and subsequent disability. Furthermore, a medical computed tomography or CBCT scan (rather than a two-dimensional panoramic radiograph) is critical for evaluation of potential MRONJ lesions. Additionally, the current scheme of management does not account for microbial assessment via cultures, which could be considered for antibiotic sensitivity testing. This patient was prescribed a broad-spectrum systemic antibiotic only when exudate was noted clinically.
Minimally invasive sequestrectomy was done for this patient as needed at each appointment based on the clinical mobility of the exposed segment and to allow for soft tissue epithelization. Certain cases may merit a total debridement based on the extent of clinical involvement. Other treatments have been suggested, such as the use of pentoxifylline and tocopherol for noninvasive management of MRONJ; however, this is presently only supported by small sample size case series.12 Furthermore, hyperbaric oxygen, which is commonly used for osteoradionecrosis, has not been proven to significantly improve healing of MRONJ lesions when compared to standard therapy with antiseptics, antibiotics and surgical debridement.13
Risk Assessment and Prevention: There is abundant evidence of the benefits of implementing dental screening and preventive treatment prior to antiresorptive therapy in reducing the risk of MRONJ.14–16 Therefore, a comprehensive oral examination, patient counselling and prophylactic care should be adopted by all centers providing oncology and osteoporosis care.17 Due to the scarcity of integrated dental services with physician care in smaller medical centers and hospitals, there is a need for development of dental-medical care models that allow better access to private dental offices for such treatments.
As this patient indicated, he did not receive counselling prior to denosumab therapy. In such instances, it is perceived as highly probable that the unaware patient may forego this information in her/his routine medical history at a dental office. For oral healthcare professionals without complete access to patients’ health records, it is imperative to take a detailed medical history for all cancer patients. Generally, patients are aware of their cancer and metastasis diagnoses, even if they are not as informed about their medications. Therefore, understanding common malignancies that affect the bone is essential.
Furthermore, understanding the half-life of denosumab (approximately 26 to 28 days), bisphosphonates (more than 10 years), and other drugs is important when determining the safety of any surgical dental procedures.18 The minimum drug holiday for denosumab should be six to eight weeks,2 which was not provided prior to dental extraction for this patient. Despite reports concerning the safety of dental procedures after lower cumulative doses of antiresorptive therapy, the additional risk from confounding systemic comorbidities and other chemotherapy medications is not always quantifiable. While restorative procedures are largely considered to be risk-free, the safety and success of surgical procedures — such as dental implants, bone grafting, periodontal surgery and laser therapy, among others — are not well known. Patients with high dose bone targeted therapy should be considered as high-risk for MRONJ.19 For any concerns, dental providers should consult the patient’s oncologist to determine the safety of a proposed surgical procedure.
REFERENCES
- National Cancer Institute. Targeted Cancer Therapies 201. Available at: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet#q4. Accessed January 14, 2021.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw — 2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956.
- Nifosi AF, Zuccarello M, Nifosi L, Hervas Saus V, Nifosi G. Osteonecrosis of the jaw in the era of targeted therapy and immunotherapy in oncology. J Korean Assoc Oral Maxillofac Surg. 2019;45:3–8.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822.
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139.
- Srivastava A, Nogueras Gonzalez GM, Geng Y, et al. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: systematic review and meta-analysis. Support Care Cancer. Epub ahead of print November 15, 2020.
- Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347.
- Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol. 2014;19:403–410.
- Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701.
- Rugani P, Walter C, Kirnbauer B, Acham S, Begus-Nahrman Y, Jakse N. Prevalence of medication-related osteonecrosis of the jaw in patients with breast cancer, prostate cancer, and multiple myeloma. Dent J (Basel). 2016;4:32.
- Cavalcante RC, Tomasetti G. Pentoxifylline and tocopherol protocol to treat medication-related osteonecrosis of the jaw: a systematic literature review. J Craniomaxillofac Surg. 2020;48:1080–1086.
- Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2017;10:CD012432.
- Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117–210.
- Bonacina R, Mariani U, Villa F, Villa A. Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: a review of 282 patients. J Can Dent Assoc. 2011;77:b147.
- Vandone AM, Donadio M, Mozzati M, et al. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience. Ann Oncol. 2012;23:193–200.
- Poxleitner P, Engelhardt M, Schmelzeisen R, Voss P. The prevention of medication-related osteonecrosis of the jaw. Dtsch Arztebl Int. 2017;114:63–69.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692.
- Stavropoulos A, Bertl K, Pietschmann P, Pandis N, Schiodt M, Klinge B. The effect of antiresorptive drugs on implant therapy: systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 18):54–92.
From Decisions in Dentistry. February 2021;7(2):32–35.




