
The Ongoing Battle Against Inflammation and Biofilm
Effective treatment of periodontal diseases requires diligent self-care and repeated professional interventions to manage inflammation and prevent bacterial recolonization.
Periodontal diseases are a worldwide public health burden.1 Undiagnosed, untreated, or inappropriately treated periodontitis can have devastating effects on oral health, quality of life, and overall health. The destructive effects and systemic impacts of periodontitis are the result of inflammation. Oral inflammation can cause tooth loss and has been linked to numerous systemic health problems. Inflammation results from the interaction of a host’s immune system and dysbiotic subgingival biofilms.
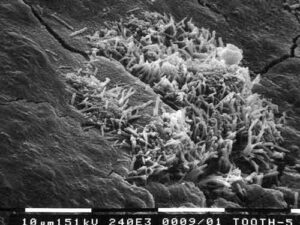
Dysbioses are problematic disruptions of normal flora. Figure 1 and Figure 2 are scanning electron micrographs depicting typical subgingival biofilm. Dysbioses seem initiated by the interactions of certain microbes, such as Porphyromonas gingivalis and Filifactor alocis.2,3 Alternately Bartold and Van Dyke4 proposed that inflammation itself is responsible for controlling the composition of biofilms. Hajishengallis5 also discussed the concept that inflammation supports the growth of dysbiotic microbial communities. Regardless of which hypothesis is correct, dysbiotic microbial communities coupled with the host’s inflammatory response can result in degradation of the periodontium.
The traditional first step in periodontal therapy involves eliminating subgingival biofilm and calculus to resolve periodontal inflammation. Success requires effective self-care and thorough subgingival debridement — including scaling and root planing (SRP) — that may need to be repeated over time.

The goal of SRP is to remove subgingival calculus and biofilm.6 Numerous studies have confirmed the significant reduction of subgingival bacteria following SRP.7 Smooth or burnished calculus, circuitous periodontal pockets, irregular roots, and a lack of visibility make SRP a demanding procedure for even accomplished clinicians.
When performed effectively, SRP can reduce subgingival biofilm, clinical inflammation, and probing depths. However, studies evaluating SRP effectiveness indicate that many teeth exhibit residual subgingival biofilm and calculus.8 Deeper probing depths, root concavities, grooves, restorative contours and furcation involvements reduce efficacy. As seen in Figure 3, the cementoenamel junction is a common site for residual calculus. No specific type of instrumentation has demonstrated consistent superiority, be it manual, ultrasonic/sonic, or lasers.9 The most important factors affecting SRP efficacy are operator experience, skill, and training.

No treatments can completely eradicate all bacteria. Limited visibility, restricted access, tissue invasion of certain microbes, and inaccessible biofilm retained in surface irregularities all impact the effectiveness of treatment.10 Previously treated sites may also be reinfected by bacteria from untreated sites, saliva, epithelium of the periodontal pocket wall and/or oral and pharyngeal mucosa, and even other individuals.11,12
Bacterial recolonization begins almost immediately following subgingival instrumentation.13,14 Post-treatment bacterial counts may reach pretreatment levels within a week.15 Initially, nonpathogenic microbes may replace periodontal pathogens.15–17 The intervals necessary for sites to return to pretreatment levels of subgingival microflora depend on disease severity, thoroughness of debridement, and supportive care.17 Thus, preventing the rebound of pathogenic bacteria requires meticulous self-care and repeated removal of subgingival biofilms at appropriate intervals.
References
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in U.S. adults. National Health and Nutrition Examination Survey 2009–2014. J Am Dent Assoc. 2018;147:576–588.
- Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Molec Med. 2015;21:172–183.
- Aruni AW, Mishra A, Dou Y, Chioma O, Hamilton BN, Fletcher HM. Filifactor alocis— a new emerging periodontal pathogen. Microbes Infect. 2015;17:517–530.
- Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol. 2019;46:6–11.
- Hajishengallis G. The inflammophilic character of the periodontitis associated microbiota. Mol Oral Microbiol. 2014;29:248–257.
- Ishikawa I, Baehni P. Nonsurgical periodontal therapy — where do we stand now? Periodontol 2000. 2004;36:9–13.
- Smiley CJ, Tracy SL, Abt E, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–524.
- Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):92–102.
- Cobb CM. Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000. 2017;75:205–295.
- Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol 2000. 2004;36:121–145.
- Quirynen M, De Soete M, Dierickx K, van Steenberghe D. The intra-oral translocation of periodontopathogens jeopardizes the outcome of periodontal therapy. A review of the literature. J Clin Periodontol. 2001;28:499–507.
- Greenstein G, Lamster I. Bacterial transmission in periodontal diseases: A critical review. J Periodontol. 1997;68:421–431.
- Magnusson I, Lindhe J, Yoneyama T, Liljemberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11:193–207.
- Cugini MA, Haffajee AD, Smith C, Kent RL, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12 month results. J Clin Periodontol. 2000;27:30–36.
- Petersilka GJ, Ehmke B, Flemmig TF. Antimicrobial effects of mechanical debridement. Periodontol 2000. 2002;28:56–71.
- Harper DS, Robinson PJ. Correlation of histometric, microbial, and clinical indicators of periodontal disease status before and after root planing. J Clin Periodontol. 1987;14:190–196.
- Shiloah J, Patters MR. Repopulation of periodontal pockets by microbial pathogens in the absence of supportive therapy. J Periodontol. 1996;67:130–139.
This information originally appeared in Rethman MP, Cobb CM, Sottosanti JS, Sheldon LN, Harrel SK. Scaling and root planing remain key to successful periodontal therapy. Decisions in Dentistry. 2021;7(9):25-31.


