Risk Assessment for Obesity and Periodontal Disease
This easily implemented strategy will assist oral health professionals in identifying patients who are at risk for inflammatory-driven conditions.
PURCHASE COURSE
This course was published in the June 2017 issue and expires June 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be able to:
- Define the concept of cumulative inflammatory burden and its relationship to systemic and oral diseases.
- Discuss the prevalence of obesity and risk of inflammatory-driven diseases, including comorbid conditions and periodontitis.
- Explain the relationship between metabolic syndrome (MetS) and periodontal disease, and a potential assessment tool for MetS risk.
The significant cost associated with delayed intervention of chronic diseases caused by systemic inflammation is widely recognized.1,2 In light of this, the authors propose a model of risk assessment that will provide an easily implemented stratagem to assist oral health care providers (OHCPs) in identifying patients at risk for inflammatory-driven comorbid conditions. This model is predicated on the recognition that the cumulative inflammatory burden associated with periodontal disease in an obese individual (defined as a body mass index ≥ 30 kg/m2; > 30% body fat in women or > 25% body fat in men) may foretell the presence of metabolic disorders, or downstream precipitation of incident diabetes, cardiovascular disease and other inflammatory-driven conditions. Better patient outcomes — and more efficient use of health care resources — may be realized by implementing this risk assessment model in dental practice.
Much has been written about systemic inflammation and its association with serious comorbid diseases.3,4 From research has come an understanding of how genetics can modify inflammatory response and the clinical expression of a number of diseases, including coronary artery disease, Alzheimer’s and periodontitis.5 Many sources elicit a state of chronic inflammation and contribute to cumulative inflammatory burden. For example, an imbalance in the intake of omega-6 and omega-3 fats,6 a diet high in red meat, processed foods, refined carbohydrates and saturated fatty acids, food sensitivities,7 psychosocial stressors, smoking,8 lack of quality sleep,9 environmental toxins,10 and infection and obesity11 all have the potential to add to the inflammatory burden, ultimately promoting cardiometabolic risk. There is a strong rationale for implementing a risk assessment model that considers patients’ cumulative inflammatory burden — which may be amplified by obesity and chronic infection from periodontal disease.
A NEW RISK ASSESSMENT MODEL
In white adipose tissue (fat cells), there is a moderate, but continuous increase in the release of a “cocktail” of inflammatory factors that are systemically dumped, including TNF-α, and IL-6 cytokines.12 Hence, obesity is widely recognized as a state of low-grade inflammation,11 fueling the risk for many diseases, including type 2 diabetes,13 hypertension,14 dyslipidemia,15 coronary artery disease,16 stroke,17 gallbladder disease,18 osteoarthritis,19 sleep apnea and pulmonary dysfunction,20 certain types of cancer21–23 and reproductive abnormalities.24
Periodontal infection, another source of low-grade inflammation, is also fueled by cytokines that mediate inflammation.12 If periodontitis is left untreated, ongoing low-grade bacteremia is produced — and, like obesity, it adds to the cumulative inflammatory burden.25–27 The greater the burden, the greater the risk and incidence of inflammatory-driven disease.
Over the last decade, mounting evidence suggests obesity is also a risk factor for periodontal disease,28–37 independent of other factors, such as age, gender, race and ethnicity; furthermore, smoking and insulin resistance may help regulate this relationship.38 In a multicentered, randomized controlled trial that studied the effects of periodontal treatment on secondary prevention of cardiovascular disease, Offenbacher et al39 demonstrated that obesity may exert such a dominant influence that when obese individuals undergo periodontal therapy, its presence may nullify the effects of periodontal treatment on high-sensitivity C-reactive protein (hs-CRP), a sensitive marker of systemic inflammation. In other words, an obese patient who undergoes therapy for periodontitis may not be able to realize the full systemic benefits of that treatment.
Studies have suggested a correlation between the severity of obesity and progression of periodontal disease. Two of the most noteworthy are the work of Gorman et al40 and Reeves et al.41 Gorman and colleagues monitored 1038 non-Hispanic white males over 25 years, and found the following:
- For each unit increase in body mass index, there was a 5% increase in the hazard of experiencing progression of alveolar bone loss.
- A 1-centimeter increase in waist circumference was associated with a 1% to 2% increase in the risk of experiencing progression of probing pocket depth and clinical attachment loss.
- With each 1% increment in baseline waist-height ratio, there was a 3% increase in the risk of experiencing periodontal disease progression (i.e., alveolar bone loss, probing pocket depth increases and clinical attachment loss).40
The study by Reeves and colleagues of 2452 U.S. adolescent nonsmokers (ages 17 to 21) noted the following:
- With each 1-kg increase in weight, there was a corresponding 6% increase in the risk of periodontal disease.
- For every 1-cm increase in waist circumference, there was a 5% increase in risk of periodontitis.41
We have hypothesized the presence of obesity and periodontal disease may be additive and therefore, in combination, confer a greater cumulative burden of inflammation;42 however, to date, only a limited number of studies have explored this matter. In a cross-sectional study, Zimmerman et al43 observed that compared to obese patients who did not have periodontal disease, leptin and IL-6 levels were elevated in the serum of individuals who were obese and had periodontal disease. These markers did not seem to be affected by scaling and root planing, suggesting that obesity may modulate local and systemic adipokines toward a proinflammatory state, regardless of periodontal treatment.44 Nevertheless, aspects related to periodontal disease severity, number of teeth affected, study design and sample size may have had an impact on these outcomes.
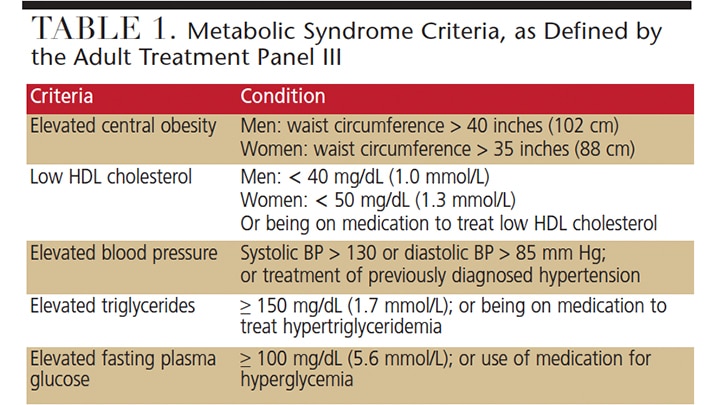
Since the 2004 paper by Pozharitskaia et al,45 there have been numerous investigations into the interrelationship between metabolic syndrome (MetS) and periodontal disease. MetS is defined as a group of specific metabolic abnormalities that, when combined, imparts a synergistic, enhanced risk for coronary artery disease, stroke and cardiovascular mortality — an effect that is more pronounced than the individual metabolic components impart alone, (e.g., hypertension or abdominal obesity).46 As one of the leading causes of death in obese individuals, MetS has a number of definitions; for the purposes of this paper, the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III or ATPIII) defines a patient with MetS as exhibiting three or more of the five criteria listed in Table 1.
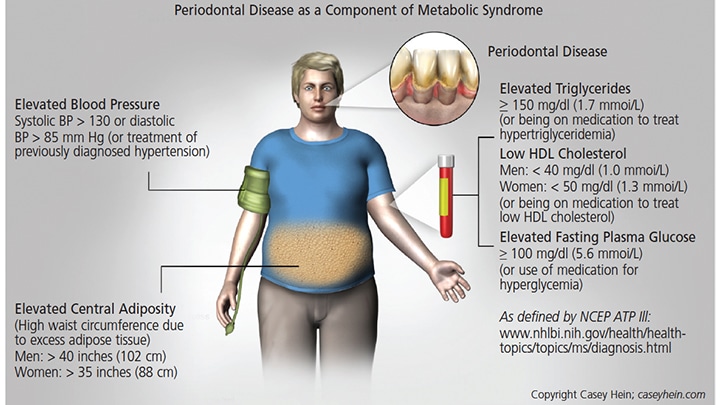
Evidence of the association between MetS and periodontal disease has been consistent enough that a number of researchers have suggested periodontal disease should be considered a component of MetS47 (Figure 1). In addition, mounting evidence suggests that MetS is positively associated with periodontal disease,25,37,47–58 as various studies have concluded
- There is an association between periodontal disease and MetS, independent of other risk factors.31
- Compared to individuals without MetS, the severity and extent of periodontal disease is significantly higher in subjects with MetS.35
- Periodontal therapy may significantly modulate hs-CRP, total leukocytes, serum triglycerides and HDL cholesterol, which may benefit individuals affected with MetS and advanced periodontal disease.59
Many individuals have MetS that has not been diagnosed, and thus have not been counseled about the risks inherent to this condition. Given the frequency that patients see their dentists and dental hygienists, OHCPs are well positioned to identify patients who have central adiposity (a telling component of MetS), and initiate discussions about cardiometabolic risk. When a patient enters the operatory, the simple task of observing his or her physique can provide valuable clues in identifying whether that individual may be at risk for MetS.
Visceral fat distribution in individuals with an apple-shaped physique, in which weight is concentrated above the waist (Figure 2), is often a sign of MetS.60 This physique, also known as android, is linked to various components of MetS, including hypertension, hypertriglyceridemia, low HDL cholesterol and insulin resistance. It is important to note that patients who present with this body type may be at increased risk for periodontal disease. For example, recent evidence from a survey that included 1287 dentate, nonsmoking and nondiabetic subjects showed that individuals who present with higher waist circumference and waist-to-height ratio were associated with an increased number of deeper pocket probing sites.61
In contrast, patients who have more weight concentrated below their waist (peripheral adiposity or a pear-shaped physique; Figure 2) have a body type that is not usually related to metabolic abnormalities. Generally, these individuals are at less risk for MetS.
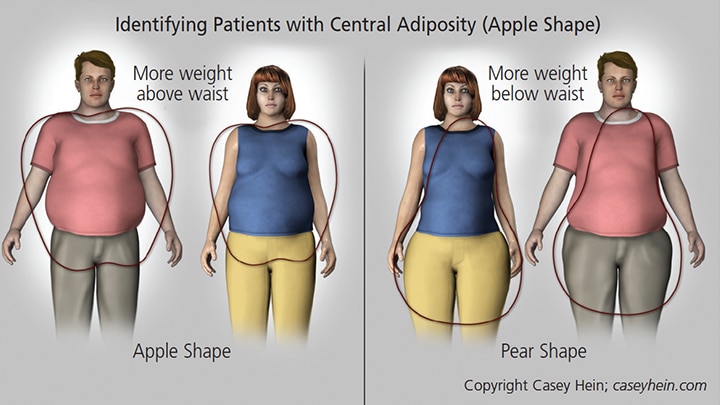
are at less risk.
UNDERLYING PRECEPTS
In recognition of the possibility that the combination of obesity and/or MetS and periodontal disease may amplify a patient’s cumulative burden of inflammation, we introduce two precepts that underpin this proposed risk assessment model.
Inflammatory Priming — Compared to individuals of normal weight, patients who are overweight or obese are “inflammatory primed,” which increases risk for inflammatory-driven diseases, including periodontal disease and concomitant systemic sequalea known to be linked to obesity. Because of their overweight or obese status, the question becomes, “What additional systemic impact will periodontal infection contribute to the cumulative inflammatory burden?”
Inflammatory Loading — Once periodontal infection is established in patients who are overweight or obese, a biological cascade of events produces proinflammatory cytokines that could also promote systemic inflammation,62,63 increasing the risk of cardiometabolic diseases. In addition, periodontal disease may exacerbate existing inflammatory-driven diseases; over time, this results in “inflammatory loading.” For example, in an obese individual, periodontal disease may elicit complications in type 2 diabetes64 or negatively influence the clinical outcome in cases of coronary artery disease in patients who do not have diabetes.
PANDEMIC OF OBESITY
One of the most ominous threats to the viability of a robust and effective health care system is the pandemic of overweight and obese individuals. Finkelstein et al65 projected a 33% increase in obesity prevalence in the United States, and a 130% increase in severe obesity over the next two decades. Given the enormity of this epidemic, its inherent costs, and the potential inflammatory priming associated with adipose tissue, it is reasonable to suggest that obesity may be the single biggest factor contributing to inflammatory-driven diseases. It is important to emphasize that periodontal disease is also associated with additional risk factors — some of which share a commonality with prevalence markers linked to MetS, such as age, socioeconomic status, smoking and ethnic background.66
Considering the emerging evidence supporting the interrelationships between obesity and periodontal disease, OHCPs can deliver more effective care by implementing a model of risk assessment that recognizes the importance of reducing patients’ cumulative inflammatory burden, especially in overweight or obese individuals with periodontal disease. This model has the potential to impact not only the periodontal health of obese and overweight patients, it could also reduce their cardiometabolic risk.
In the past, the responsibility for obesity intervention fell solely to physicians, nurses, dietitians and other nondental health care professionals. The traditional scope of practice for OHCPs has not included screening and referral of patients who are obese or at risk for obesity or MetS. However, the sheer magnitude of the obesity epidemic requires all providers to become involved.
After analyzing data collected from 2009–2010, researchers noted that more than 33% of U.S. adults and almost 17% of youth were considered obese67 — and these rates have likely risen since the study. Superimposing these statistics onto the patient base of the average general dental practice should elicit a call to action. In 2008, at least 70% of Americans age 18 and older visited a dentist,68 thus, OHCPs are in a unique position to address obesity in their patients, especially in children and adolescents.
It is imperative that providers from all health care disciplines work together at the point of care to assess patients for the presence of chronic, low-grade inflammation. This must be bilaterally implemented; dentists and dental hygienists should move beyond identifying periodontal disease (or other oral health issues) in isolation of assessing risk for whole-body, inflammatory-driven comorbid diseases. It is equally important for nondental providers to recognize that periodontal disease may amplify the cumulative inflammatory burden in a patient who is obese or diagnosed with an inflammatory-driven disease.
FINAL REMARKS
It is important for clinicians, health care educators, public health authorities and policymakers to be informed of the potential surge in oral health issues that will likely accompany increases in the population of obese individuals, and the concomitant risks associated with inflammatory priming in this population. Accordingly, it is essential to develop protocols and clinical tools that will direct care in the individualized management and prevention of oral diseases in patients with metabolic diseases.
Close monitoring of the periodontal parameters of obese patients is of obvious import; however, protocols and clinical tools to assist clinicians in educating patients about the significance of lifestyle modification are essential. These include educational counseling, nutritional assessment and dietary treatment planning to help reduce a hyperresponsive inflammatory trait, among other clinical measures.
Although somewhat controversial, the concept of utilizing OHCPs to identify patients who have central adiposity offers promise in mitigating the risk for obesity and preventing metabolic diseases, in addition to benefitting these patients’ periodontal health. Adoption of this new model of risk assessment has the potential to modify the trajectory of inflammatory-driven diseases, sustaining health and, ultimately, prolonging life.
REFERENCES
- Lakdawalla DN, Goldman DP, Shang B. The health and cost consequences of obesity among the future elderly. Health Aff(Millwood). 2005;24(Suppl 2):W5R30–W5R41.
- Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52–61.
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature Rev Immunol. 2011;11:98–107.
- Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79:1544–1551.
- Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am J Clin Nutr. 2006;83:475S–483S.
- Adamo AM. Nutritional factors and aging in demyelinating diseases. Genes Nutr. 2014;9:360.
- Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–149.
- Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Medicine. 2013;11:200.
- Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102.
- Hope J. A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. ScientificWorldJournal. 2013;2013:767482.
- Cancello R, Clément K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141–1147.
- Khosravi R, Ka K, Huang T, et al. Tumor necrosis factor-α and interleukin-6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013:728987.
- Connolly VM, Gallagher A, Kesson CM. A study of fluoxetine in obese elderly patients with type 2 diabetes. Diabet Med. 1995;12:416–418.
- Reisin E, Frohlich ED, Messerli FH, et al. Cardiovascular changes after weight reduction in obesity hypertension. Ann Intern Med. 1983;98:315–319.
- Denke MA, Sempos CT, Grundy SM. Excess body weight. An under-recognized contributor to dyslipidemia in white American women. Arch Intern Med. 1994;154:401–410.
- Shekelle RB, Shryock AM, Paul O, et al. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N Engl J Med. 1981;304:65–70.
- Shaper AG, Wannamethee SG, Walker M. Body weight: implications for the prevention of coronary heart disease, stroke, and diabetes mellitus in a cohort study of middle aged men. BMJ. 1997;314:1311–1317.
- Haffner SM, Diehl AK, Stern MP, Hazuda HP. Central adiposity and gallbladder disease in Mexican Americans. Am J Epidemiol. 1989;129:587–595.
- Felson DT. Weight and osteoarthritis. J Rheumatol Suppl. 1995;43:7–9.
- Gold AR, Schwartz AR, Wise RA, Smith PL. Pulmonary function and respiratory chemosensitivity in moderately obese patients with sleep apnea. Chest. 1993;103:1325–1329.
- Chu SY, Lee NC, Wingo PA, Senie RT, Greenberg RS, Peterson HB. The relationship between body mass and breast cancer among women enrolled in the Cancer and Steroid Hormone Study. J Clin Epidemiol. 1991;44:1197–1206.
- Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control. 1996;7:253–263.
- Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110–132.
- Kaye SA, Folsom AR, Prineas RJ, Potter JD, Gapstur SM. The association of body fat distribution with lifestyle and reproductive factors in a population study of postmenopausal women. Int J Obes. 1990;14:583–591.
- D’Aiuto F, Sabbah W, Netuveli G, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008;93:3989–3994.
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227.
- Zhang W, Daly CG, Mitchell D, Curtis B. Incidence and magnitude of bacteraemia caused by flossing and by scaling and root planing. J Clin Periodontol. 2013;40:41–52.
- Al-Zahrani MS, Bissada NF, Borawskit EA. Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol. 2003;74:610–615.
- Alabdulkarim M, Bissada N, Al-Zahrani M, Ficara A, Siegel B. Alveolar bone loss in obese subjects. J Int Acad Periodontol. 2005;7:34–38.
- Buhlin K, Gustafsson A, Pockley AG, Frostegård J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099–2107.
- Nishida N, Tanaka M, Hayashi N, et al. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. J Periodontol. 2005;76:923–928.
- Nishimura F, Kono T, Fujimoto C, Iwamoto Y, Murayama Y. Negative effects of chronic inflammatory periodontal disease on diabetes mellitus. J Int Acad Periodontol. 2000;2:49–55.
- Perlstein MI, Bissada NF. Influence of obesity and hypertension on the severity of periodontitis in rats. Oral Surg Oral Med Oral Pathol. 1977;43:707–719.
- Saito T, Shimazaki Y, Kiyohara Y, et al. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: the Hisayama study. J Periodontal Res. 2005;40:346–353.
- Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. J Dent Res. 2001;80:1631–1636.
- Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med. 1998;339:482–483.
- Wood N, Johnson RB, Streckfus CF. Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III). J Clin Periodontol. 2003;30:321–327.
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084.
- Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201.
- Gorman A, Kaye EK, Apovian C, Fung TT, Nunn M, Garcia RI. Overweight and obesity predict time to periodontal disease progression in men. J Clin Periodontol. 2012;39:107–114.
- Reeves AF, Rees JM, Schiff M, Hujoel P. Total body weight and waist circumference associated with chronic periodontitis among adolescents in the United States. Arch Pediatr Adolesc Med. 2006;160:894–899.
- Hein C, Batista EL. Obesity and cumulative inflammatory burden: A valuable risk assessment parameter in caring for dental patients. J Evid Based Pract. 2014;14:17–26.
- Zimmermann GS, Bastos MF, Dias Gonçalves TE, Chambrone L, Duarte PM. Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. J Periodontol. 2013;84:624–633.
- Gonçalves TED, Zimmermann GS, Figueiredo LC, et al. Local and serum levels of adipokines in patients with obesity after periodontal therapy: one-year follow-up. J Clin Periodontol. 2015;42:431–439.
- Pozharitskaia MM, Simakova TG, Starosel’tseva LK, Kirienko VV. [Inflammatory diseases of the parodontium in patiens with metabolic syndrome]. Stomatologiia (Mosk). 2004;83:13–16.
- Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375.
- Nishimura F, Soga Y, Iwamoto Y, Kudo C, Murayama Y. Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol. 2005;7:16–20.
- Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37:601–608.
- Borges PK, Gimeno SG, Tomita NE, Ferreira SR. Prevalence and characteristics associated with metabolic syndrome in Japanese-Brazilians with and without periodontal disease. Cad Saude Publica. 2007;23:657–668.
- Han DH, Lim SY, Sun BC, Paek D, Kim HD. The association of metabolic syndrome with periodontal disease is confounded by age and smoking in a Korean population: the Shiwha-Banwol Environmental Health Study. J Clin Periodontol. 2010;37:609–616.
- Khader Y, Khassawneh B, Obeidat B, et al. Periodontal status of patients with metabolic syndrome compared to those without metabolic syndrome. J Periodontol. 2008;79:2048–2053.
- Kushiyama M, Shimazaki Y, Yamashita Y. Relationship between metabolic syndrome and periodontal disease in Japanese adults. J Periodontol. 2009;80:1610–1615.
- Kwon YE, Ha JE, Paik DI, Jin BH, Bae KH. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol. 2011;38:781–786.
- Li P, He L, Sha YQ, Luan QX. Relationship of metabolic syndrome to chronic periodontitis. J Periodontol. 2009;80:541–549.
- Morita T, Yamazaki Y, Mita A, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512–519.
- Nibali L, D’Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007;34:931-937.
- Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res. 2007;86:271–275.
- Timonen P, Niskanen M, Suominen-Taipale L, Jula A, Knuuttila M, Ylöstalo P. Metabolic syndrome, periodontal infection, and dental caries. J Dent Res. 2010;89:1068–1073.
- Acharya A, Bhavsar N, Jadav B, Parikh H. Cardioprotective effect of periodontal therapy in metabolic syndrome: a pilot study in Indian subjects. Metab Syndr Relat Disord. 2010;8:335–341.
- Lebovitz HE. The relationship of obesity to the metabolic syndrome. Int J Clin Pract Suppl. 2003:18–27.
- Kangas S, Timonen P, Knuuttila M, Jula A, Ylöstalo P, Hannele Syrjälä AM. Waist circumference and waist-to-height ratio are associated with periodontal pocketing — results of the Health 2000 Survey. BMC Oral Health. 2017;17:4
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–2100.
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115.
- D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40(Suppl 14):S85–S105.
- Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570.
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon, MR, Heymsfield SB. The metabolic syndrome prevalence and associated risk factor findings in the U.S. Population from the Third National Health and Nutrition Examination Survey, 1988–1994 Arch Intern Med. 2003;163:427–436.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8.
- U.S. Centers for Disease Control and Prevention. National Oral Health Surveillance System. Available at: cdc.gov/oralhealthdata/overview/nohss.html. Accessed May 16, 2017.
Featured Image by STEVE COLE IMAGES/ISTOCK/GETTY IMAGES PLUS
From Decisions in Dentistry. June 2017;3(6):42—46.