
Diagnosing and Treating Dentin Hypersensitivity
An examination of predisposing factors, differential diagnosis and therapies for dentin hypersensitivity.
This course was published in the October 2018 issue and expires October 2021. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the prevalence of dentin hypersensitivity, as well as possible etiologies.
- Explain the diagnostic process for this condition.
- Describe management strategies for this patient population.
Dentin hypersensitivity is one of the most common painful conditions affecting oral comfort and function.1 The Canadian consensus document has defined it as “pain derived from exposed dentin in response to chemical, thermal, tactile or osmotic stimuli which cannot be explained as arising from any other dental defect or disease.2 It may affect up to 85% of the population.3 The disease is common in patients age 20 to 50, but becomes more prevalent in patients from age 30 to 40, particularly women.4 Among periodontal patients, the occurrence of cervical dentin sensitivity ranges between 72% to 98%.5 This condition occurs most frequently in canines and premolars, and often involves buccal surfaces.6
Besides dentists, dental hygienists play an important role in the prevention and treatment of dentin hypersensitivity by providing nonsurgical periodontal therapy, placing desensitizing materials, and emphasizing good hygiene practices. Moreover, dental hygienists may be the first to recognize the condition in patients who experience discomfort during prophylaxis appointments. As part of the health history, providers should inquire about any dental concerns, including sensitivity or possible behavior modifications that patients are using to evade pain.7,8
This condition is diagnosed by eliminating other possible causes, such as gingival inflammation, caries, cracked tooth syndrome, broken restorations, marginal leakage, postoperative sensitivity, pulpitis or sensitivity from tooth bleaching.9 Several theories have been advanced to explain the mechanism of dentin hypersensitivity.1 The concept with the most acceptance is hydrodynamic theory, which states that, following stimulus, shifts of fluids within dentinal tubules activate sensory nerves in the pulp and inner dentin.10,11
The pulpodentin complex is innervated by mylenated (Aβ and Aδ) and ummylenated C-fiber sensory nerves. Sensitivity results from the activation of Aβ and Aδ sensory nerves in dentinal tubules adjacent to the dentin-pulp junction. Certain stimuli can result in a pressure modification across the dentin, resulting in excitation of individual intradental nerves. The response seems to be proportional to pressures that increase fluid flow.12 Cold that results in fluid flow away from the pulp demonstrates increased nerve response, while heat (which causes an inward flow) demonstrates a reduced response. The stimuli resulting in pain can be thermal, tactile, osmotic, chemical or evaporative, but cold is usually the most problematic.13 Clinicians will note differences between hypersensitive and nonsensitive dentin, as there are more and wider tubules in sensitive dentin.14
GINGIVAL RECESSION AND VITAL BLEACHING
Gingival recession often precedes dentin hypersensitivity and is one of most important factors in this condition.5,15,16 Loss of cervical enamel and dentin due to aggressive toothbrushing (in conjunction with more abrasive dentifrices,17–19 and especially after erosive food and drink) is also a contributing factor. Although gingival recession is preventable, predisposing factors include root prominence with a thin biotype, dehiscences and fenestrations in the bone under the gingiva, frenum pulls, and orthodontic movement of roots outside the bony housing.15 Some patients may also have imperfect or partially absent cementum construction. Poor oral hygiene can also be a factor.20 Inflammatory periodontitis eventually causes facial lingual bone and gingiva to recede, potentially leading to increased hypersensitivity.21 Periodontal surgery may also result in exposure of root surfaces and increased sensitivity.22
While tooth bleaching is a common cosmetic procedure,23 whitening agents can penetrate tooth structure and release oxygen radicals that are capable of oxidizing chromogens. In-office whitening may result in dentin hypersensitivity as a result of oxidative stress caused by hydrogen peroxide and its associated hydroxyl radical formation.24 Home bleaching can also lead to sensitivity.25,26 Analgesics, anti-inflammatories, antioxidants and corticosteroids have been proposed prior to bleaching to reduce side effects, but have not proven effective. Application of glutaraldehyde-based products has shown efficacy in reducing sensitivity prior to bleaching.27 Beyond these approaches, potassium nitrate, fluorides and remineralizing materials have also been used — though with mixed results
EROSION AND ABFRACTION
Defined as chemical wear resulting from extrinsic or intrinsic acid or chelators on plaque-free tooth surfaces,28 erosion from extrinsic acids is a key factor in tooth wear. Patients with cervical wear, for example, often present with dentin hypersensitivity.29 According to Grippo et al,30 a paradigm shift is suggested to use the term “biocorrosion” to supplant “erosion.” Biocorrosion embraces the chemical, biochemical, and electrochemical degradation of tooth substance caused by endogenous and exogenous acids, proteolytic agents, as well as the piezoelectric effects only on dentin.30
Abfraction, representing the microstructural loss of tooth substance in areas of stress concentration, should not be used to designate all noncarious cervical lesions (NCCLs) because these lesions are commonly multifactorial in origin. Appropriate designation of an NCCL depends on the interplay of stress, friction and biocorrosion unique to that individual case.30 Abfraction is believed to be the consequence of tensile or shear stress in the cementoenamel junction, resulting in microfractures in enamel and dentin.31,32 It is also thought that Hunter-Schraeger bands in this susceptible area may result in abfraction.33 Although occlusal trauma is thought to cause these lesions, some teeth, although in traumatic occlusion, don’t exhibit such lesions — therefore, its etiology may be multifactorial.
TREATMENT OPTIONS
Clinicians need to determine the cause of sensitivity in order to present the best treatment options (Table 1). Toward this goal, the patient’s response to a variety of triggering stimuli — mechanical, chemical, electrical and thermal34,35 — should be recorded. Management can involve either in-office or at-home therapy (or a combination), and these options fall under two main categories: nerve stabilization/desensitization or occlusion.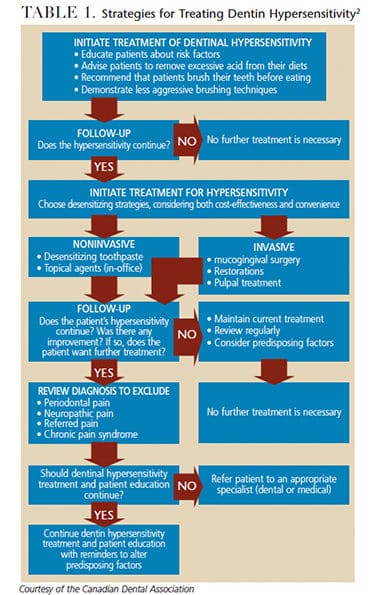
Desensitizing agents may be found in certain self-care products, including tooth powders, toothpastes, mouthrinses and chewing gum. While many desensitizing toothpastes use potassium salts, such as potassium chloride, potassium citrate and potassium nitrate,36 alternative agents may include strontium acetate, arginine and calcium carbonate, calcium sodium phosphosilicate, fluoride and other formulations.
In high concentrations, fluoride can be effective in reducing sensitivity through application of gels and varnishes. Available in self-care products or professionally applied prophylaxis pastes and varnishes, additional therapies include calcium phosphate technologies — such as amorphous calcium phosphate, casein phosphopeptide/amorphous calcium phosphate, and calcium sodium phosphosilicate. Another calcium phosphate technology, tricalcium phosphate, provides a slow release of calcium to the tooth surface that is designed to boost the remineralizing effects of fluoride, which may also decrease sensitivity.37 Other desensitizing products use calcium carbonate to form occlusion with arginine to block tubules.38–40
Offered for in-office and at-home treatment, oxalates have been used to block dentinal fluid flow by occluding tubules. Barrientos et al41 found teeth treated with an oxalate compound demonstrated significantly less sensitivity after four months. They also have the advantage of being relatively insoluble in acids and resistant to dissolution with saliva and brushing. Potassium oxalate causes crystal-like deposits inside the tubules and also reacts with calcium ions present in dentinal fluid to create insoluble calcium oxalate crystals.42 In addition to tubule-occluding properties, potassium oxalate may cause inhibition of intradental nerves. This may be the result of increased potassium ion concentration causing depolarization of the nerves by means of a process termed axonal accommodation.43,44 Also available is an oxalic acid, potassium salt and water (potassium oxalate) formula that is applied on acid-etched dentin, rinsed and left moist for bonding. The material stimulates precipitation of calcium oxalate crystals inside the dentinal tubules and helps eliminate fluid movement and corresponding sensitivity.
Unlike other local desensitizing agents that have a short-term effect, adhesive systems have a long-standing or permanent effect. Yu et al45 found topical application of dentin adhesives/desensitizers on sensitive areas resulted in occlusion of the patent tubules. This relieved sensitivity immediately and over the course of a month following treatment. These adhesives include select varnishes, bonding agents and repairing resin composites. The composites can effectively seal dentinal tubules by forming a hybrid layer.29 The new adhesives act in a way that the smear layer will be modified and incorporated into the hybrid layer. Also available is a desensitizing agent that includes hydroxyethyl methacrylate (HEMA), benzalkonium chloride, glutaraldehyde and fluoride. Glutaraldehyde can lead to protein coagulation within dentinal tubules, while HEMA can cause resin tags to be formed that help occlude tubules.46 Clinicians can also choose HEMA-based desensitizing agents that do not contain glutaraldehyde, including a light-cured product.
LASER THERAPY
The effect of dental lasers for treating dentin hypersensitivity might be between 5% to 100%, depending on the type of laser and beneficial parameters, such as the laser’s length of beam, treatment time, and laser intensity.46,47 A systematic review suggests laser therapy has a slight clinical advantage over topical medicaments.48 Ko et al49 found the use of a low-level, laser-emitting toothbrush to be a safe and effective treatment for sensitivity.
Various mechanisms of action have been proposed for the laser, its effect on the dentin, and its impact on reducing sensitivity; these include:
- Occlusion through coagulation of the proteins of the fluid inside the dentinal tubules
- Occlusion of tubules through partial sub-melting
- Discharging of internal tubular nerve48
CONCLUSION
Dentin hypersensitivity is a compelling and common issue facing dental practitioners. Because other conditions may share similar symptoms, differential diagnosis is essential. This should be followed by a management plan that addresses any predisposing conditions (Table 2). It is imperative to rule out active pathologic conditions (e.g., tooth fracture or pulpal inflammation) prior to treating sensitivity.
Treatment decisions should be based on sensitivity severity and etiology. Some in-office therapies offer immediate relief that can be followed with at-home remedies. A combination of techniques may be warranted to provide long-term relief. An at-home regimen can be initiated with a variety of available materials to either block the tubules or reduce pain impulses. In many cases, this condition can be treated with desensitizing toothpastes. Patients should be advised, however, that clinical trials have demonstrated the use of desensitizing pastes often requires two to four weeks for maximum effectiveness.50 If the patient’s dentin hypersensitivity is still problematic after using a desensitizing toothpaste for an appropriate interval, clinicians should consider the variety of in-office treatments previously discussed.
REFERENCES
- Dowell P, Addy M. Dentine hypersensitivity — a review. Aetiology, symptoms and theories of pain production. J Clin Periodontol. 1983;10:341–350.
- Canadian Advisory Board on Dentin Hypersensivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226.
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367–375.
- Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–332.
- Chabanski MB, Gillam DG, Bulman JS, Newman HN. Clinical evaluation of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department: a pilot study. J Oral Rehabil. 1997;24:666–672.
- Aranha AC, Pimenta LA, Marchi GM. Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res. 2009;23:333–339.
- Schiff T, Delgado E, Zhang YP, Cummins D, DeVizio W, Mateo LR. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent. 2009;22(Spec No A):8A–15A.
- Davari AR, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent (Shiraz). 2013;14:136–145.
- Jacobsen PL, Bruce G. Clinical dentin hypersensivity: understanding the causes and prescribing treatment. J Contemp Dent Pract. 2001;2:1–12.
- Gysi A. An attempt to explain the sensitiveness of dentin. Br J Dent Sci. 1900;43:865–868.
- Brännström M. A hydrodynamic mechanism in the transmission of pain-produced stimuli through the dentin. In: Anderson DJ, ed. Sensory Mechanisms in Dentine. New York: Pergamon Press; 1962:73–79.
- Matthews B, Vongsavan N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol. 1994;39(Suppl):87S–95S.
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity: a study of the patency of dentinal tubules in sensitive and nonsensitive cervical dentine. J Clin Periodontol. 1987;14:280–284.
- Addy M. Dentin hypersensitivity: prevalence, distribution and etiology. In: Addy M, Emberry G, Edgar M, Orchardson R, eds. Tooth Wear and Sensitivity: Clinical Advances in Restorative Dentistry. London: Martin Dunitz Publishers; 2000:239–248.
- Tugnait A, Clerehugh V. Gingival recession — its significance and management. J Dent. 2001;29:381–394.
- Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53(Suppl 3):177–186.
- Drisko CH. Dentine hypersensitivity — dental hygiene and periodontal considerations. Int Dent J. 2002;52(Suppl):385–393.
- Strassler HE, Drisko CL, Alexander DC. Dentin hypersensitivity: its inter-relationship to gingival recession and acid erosion. Compend Contin Educ Dent. 2008;29:1–9.
- Smith RG. Gingival recession reappraisal of an enigmatic condition and a new index for monitoring J Clin Periodontal. 1997;24:201–205.
- Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S237–S248.
- Kassab MM, Cohen RE. The etiology and prevalence of gingival recession. J Am Dent Assoc. 2003;134:220–225.
- de Paula EA, Kossatz S, Fernandes D, Loguericio AD, Reis A. Administration of ascorbic acid to prevent bleaching-induced tooth sensitivity: a randomized triple-blind clinical trial. Oper Dent. 2014;39:128–135.
- de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA. Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig. 2017;21:2509–2520.
- Basting RT, Amaral FL, França FM, Flório FM. Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent. 2012;37:464–473.
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772.
- Mehta D, Venkata S, Naganath M, LingaReddy UIshihata H, Finger WJ. Clinical trial of tooth desensitization prior to in-office bleaching. Eur J Oral Sci. 2013;121:477–481.
- Lussi A, Jaeggi T. Erosion-diagnosis and risk factors. Clin Oral Investig. 2008;12(Suppl 1):S5–S13.
- Bartlett DW. The causes of dental erosion. Oral Dis. 1997;3:209–211.
- Grippo JO, Simring M, Coleman TA. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: a 20-year perspective. J Esthet Restor Dent. 2012;24;10–23.
- Grippo JO. Abfractions: a new classification of hard tissue lesions of teeth. J Esthet Dent. 1991;3:14–19.
- Coleman TA, Grippo JO, Kinderknecht KE. Cervical dentin hypersensitivity. Part II: associations with abfractive lesions. Quintessence Int. 2000;31:466–473.
- Lynch CD, O’Sullivan VR, Dockery P, McGillycuddy CT, Rees JS, Sloan AJ. Hunter-Schreger Band patterns and their implications for clinical dentistry. J Oral Rehabil. 2011;38:359–365.
- Trushkowsky RD, Garcia-Godoy F. Dentin hypersensitivity: differential diagnosis, tests, and etiology. Compend Contin Educ Dent. 2014;35:99–104.
- García-Godoy F, Trushkowsky RD. A diagnostic device to record dentin hypersensitivity. Am J Dent. 2013;26(Spec No B):3B–4B.
- Kwon SR, Dawson DV, Schenck DM, Fiegel J, Wertz PW. Spectrophotometric evaluation of potassium nitrate penetration into the pulp cavity. Oper Dent. 2015;40:614–621.
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically-modified calcium phosphate. J Mater Sci. 2009;44:346–349.
- Uraz A, Erol-Şimşek Ö, Pehlivan S, Suludere Z, Bal B. The efficacy of 8% arginine-CaCO₃ applications on dentine hypersensitivity following periodontal therapy: a clinical and scanning electron microscopic study. Med Oral Patol Oral Cir Bucal. 2013;18:e298–e305.
- Panagakos F, Schiff T, Guignon A. Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent. 2009;22(Suppl No A):3A–7A.
- Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;20:1–9.
- Barrientos C, Xaus G, Leighton C, Martin J, Gordan VV, Moncada G. Oxalic acid under adhesive restorations as a means to reduce dentin sensitivity: a four-month clinical trial. Oper Dent. 2011;36:126–132.
- Gillam DG, Mordan NJ, Sinodinou AD, Tang JY, Knowles JC, Gibson IR. The effects of oxalate-containing products on exposed dentin surface: an SEM investigation. J Oral Rehabil. 2001;28:1037–1044.
- Markowitz K, Bilotto G, Kim S. Decreasing intradental nerve activity in the cat with potassium and divalent cations. Arch Oral Biol. 1991;36:1–7.
- Kim SY, Kim EJ, Kim DS, Lee IB. The evaluation of dentinal tubule occlusion by desensitizing agents: a real-time measurement of dentinal fluid flow rate and scanning electron microscopy. Oper Dent. 2013;38:419–428.
- Yu X, Liang B, Jin X, Fu B, Hannig M. Comparative in vivo study on the desensitizing efficacy of dentin desensitizers and one-bottle self-etching adhesives. Oper Dent. 2010;35;279–286.
- He S, Wang Y, Li X, Hu D. Effectiveness of laser therapy and topical desensitising agents in treating dentine hypersensitivity: a systematic review. J Oral Rehabil. 2011;38:348–358.
- Lopes AO, de Paula Eduardo C, Aranha AC. Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci. 2017;32:1023–1030.
- Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod. 2011;37:297–303.
- Ko Y, Park J, Kim C, Park J, Baek SH, Kook YA. Treatment of dentin hypersensitivity with a low-level laser-emitting toothbrush: double-blind randomised clinical trial of efficacy and safety. J Oral Rehabil. 2014;41:523–531.
- Kanapka JA. Over the counter dentifrices in the treatment of tooth hypersensitivity: review of clinical studies. Dent Clin North Am. 1990;34:545–560.
Featured image by ISAYILDIZ/ISTOCK/GETTY IMAGES PLUS
From Decisions in Dentistry. October 2018;4(10):21–23.






The number one cause of gingival recession and abfraction in my practices is from bite forces. I am sad that this article does not even mention, let alone address this as a cause.
I agree with sam weiz. Both abfraction and gum recession is often due to an unstable bite