
Pain Management in Endodontic Treatment
An examination of clinical strategies for managing pain before, during and after endodontic procedures.
This course was published in the August 2017 issue and expires August 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
OBJECTIVES
After reading this course, the participant should be able to:
- Discuss strategies for managing pain in endodontic patients.
- Explain clinical considerations for pulpal diagnosis and endodontic treatment planning.
- List the various agents and drugs used in endodontic therapy and pain management.
The need to perform endodontic treatment is likely due to a patient presenting with inflamed and/or infected pulpal tissue. This pretreatment histologic pulp status can have a direct correlation to a patient’s odontogenic pain symptoms. Therefore, pain management in endodontic treatment is an important clinical consideration. Pain, as reported by a patient before, during and/or after endodontic treatment, should be managed clinically with local anesthesia, endodontic treatment and medications. This article is intended to serve as an overview of pain management strategies in endodontic treatment.
It is paramount for the clinician to obtain profound anesthesia when providing endodontic treatment. A common mistake is not objectively testing if pulpal anesthesia has been achieved prior to initiating therapy. Often, the only determination made to confirm if a patient is properly anesthetized is the subjective anesthesia level, as reported by the patient. Studies have demonstrated that, on average, inferior alveolar nerve anesthetic blocks administered to patients with mandibular teeth diagnosed with irreversible pulpitis had only a 55% incidence of profound pulpal anesthesia, even in the presence of 100% lip numbness, as reported by the patient.1,2
Therefore, prior to administering local anesthesia for endodontic treatment, the provider should objectively assess the treatment tooth (Figure 1) using a cold test and/or an electric pulp tester (EPT). With a preoperative baseline of the pulp sensibility level, once anesthesia is achieved, retesting the treatment tooth with cold or an EPT can assess the level of anesthesia. If the postanesthesia test results are negative to cold or no response to EPT, there is a high likelihood that profound pulpal anesthesia has been achieved. It is important to note, however, that teeth with metal restorations can provide a false positive test when using an EPT. In addition, a study by Fuss et al3 reported that using an EPT was less reliable than cold testing in young patients.
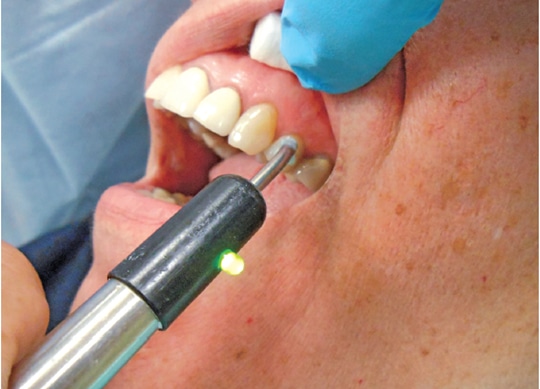
before and after administering local anesthesia to determine if pulpal anesthesia
has been achieved.
REGIONAL AND SUPPLEMENTAL LOCAL ANESTHESIA
Another common mistake when trying to achieve profound pulpal anesthesia is only giving an infiltration around the treatment tooth. Although this may prove effective for treating a small cavity, it is not advisable for endodontic treatment. The clinician should first administer a regional block for local anesthesia, using either an inferior alveolar nerve block for the mandibular region, or a superior alveolar nerve block for maxillary teeth.
If profound anesthesia cannot be achieved with a regional block alone (as determined by objective testing), supplemental anesthesia should be administered. Examples of supplemental local anesthesia injections are long buccal nerve blocks (for mandibular molars), periodontal ligament, intraosseous and intrapulpal. If supplemental anesthesia is administered prior to a regional block, it will either be short acting or not adequately effective in providing pulpal anesthesia. Of note, reinjection of local anesthesia in the same regional or supplemental site has been shown to increase success in achieving pulpal anesthesia.1
ENDODONTIC TREATMENT
Performing endodontic treatment can significantly reduce pretreatment odontogenic pain due to acute pulpal inflammation.4 Studies have demonstrated that pulpotomy and pulpectomy treatment will reduce preoperative tooth pain.5,6
A recent study by Ricucci et al7 found that a clinical pulpal diagnosis of a normal pulp or reversible pulpitis had a 96.6% histological match to the actual pulp tissue in the teeth. Therefore, patients who present with pain from a carious pulp exposure and a pulp diagnosis of reversible pulpitis can be successfully treated with a pulpotomy and mineral trioxide aggregate (MTA) if the pulp is exposed during treatment.8 A randomized clinical trial by Hilton et al9 demonstrated that MTA, a bioceramic material, performed significantly better than calcium hydroxide as a direct pulp-capping agent.
endodontic access.
Bioceramics or calcium silicate-based materials are biocompatible, nontoxic, nonshrinking and are usually stable within a biological environment. An additional advantage of bioceramic materials is their ability to form calcium hydroxide and hydroxyapatite.10 Clinicians can choose from numerous bioceramics for pulp capping, root repair and root canal obturation.
In teeth with signs and symptoms suggestive of a pretreatment pulpal diagnosis of symptomatic irreversible pulpitis, pulpal conditions have less than a 15.6% chance of reverting to normal by a pulpotomy alone; thus, complete removal of the pulp (i.e., a pulpectomy) is the treatment of choice.7 If the pretreatment pulpal diagnosis is a necrotic pulp, a pulpotomy is contraindicated, and a pulpectomy should be performed.
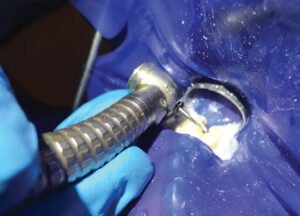
When providing endodontic treatment on posterior teeth, occlusal reduction should be performed prior to creating endodontic access. Occlusal adjustment (Figure 2) may help reduce postoperative pain in patients who present with preoperative pain, pulp vitality, percussion sensitivity and/or the absence of a periradicular radiolucency.11
INTRACANAL IRRIGANTS AND MEDICAMENTS
Conventional endodontic thinking is that files shape and irrigants clean. Root canal morphology is often complex, which makes it challenging to prepare canals by removing all vital or non-vital pulp tissue using endodontic files alone.12 A possible explanation for the inefficiency of file canal preparation is that nickel-titanium rotary files tend to stay centered in the canal, and thus may not make contact with all dentinal walls due to various invaginations and irregularities.13
As a result, employing various irrigants and medicaments is important when removing vital or necrotic pulp tissue from a canal. The use of sodium hypochlorite (NaOCl) as a canal irrigant can dissolve vital pulp tissue and disinfect a canal system,14 and it is especially important to use NaOCl irrigation when treating an inflamed vital pulp. It is also an effective irrigant when removing necrotic pulp tissue.
The use of ethylenediaminetetraacetic acid (EDTA) in conjunction with NaOCl helps in removing inflamed or infected pulpal tissue from the canal wall. This is a chelating agent (i.e., it removes calcium ions) and aids in the removal of the smear layer (residual canal debris left on the canal walls from instrumentation). Available for clinical use as a 17% solution and in gel form, the solution is used as a final rinse prior to obturation. The 17% EDTA solution can help clean the dentinal wall and expose dentinal tubules and small accessory canals. In addition, EDTA gel should be placed on every file prior to activating it in a canal (Figure). The removal of the smear layer from a canal wall will help irrigants and medicaments kill microorganisms in the dental tubules. The use of EDTA gel can also prevent dentinal chips and pulp collagen from blocking a canal to the point clinicians cannot maintain proper working length.
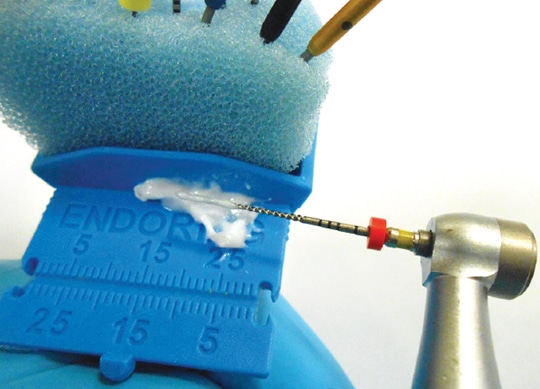
to insertion into the root canal.
Other intracanal medicaments that can reduce pain after endodontic therapy include calcium hydroxide and chlorhexidine. When compared to calcium hydroxide or the use of no medicaments, an in vivo study by Singh et al15 found that in patients with a diagnosis of necrotic pulp and acute apical periodontitis, the use of chlorhexidine alone or calcium hydroxide and chlorhexidine as an interappointment medicament reduced postoperative pain.
When treating necrotic pulps, Walton and Fouad16 demonstrated that the frequency of posttreatment flare-ups was significantly higher in necrotic pulp cases than vital cases. A flare-up is characterized by the development of pain and/or swelling following endodontic treatment. Although the etiology of posttreatment flare-ups encompasses mechanical, chemical and/or microbial agents, microorganisms are arguably the major causative factor.17 A clinical study by Yoldas et al18 reported that two-visit (compared to one-visit) treatment on teeth with necrotic pulps demonstrated a significant reduction of interappointment flare-ups. This study used an intracanal medication of calcium hydroxide and chlorhexidine for one week between the first and second appointments.
POSTTREATMENT MEDICATIONS
The most consistent predictive factor for postoperative endodontic pain is the presence of preoperative hyperalgesia (i.e., spontaneous pain, reduced pain threshold and/or increased perception to noxious stimuli).19 A clinical study by Ali et al20 showed that postoperative pain was present in 54.5% of patients treated. A common mistake when managing endodontic pain is prescribing drugs posttreatment without critically assessing if they are pharmacologically treating inflammation and/or infection. An example would be prescribing antibiotics for tooth pain that has an inflammatory, rather than an infectious, etiology. Fouad et al21 found that antibiotics do not have an analgesic effect on odontogenic inflammatory pain. A clinical guide for determining inflammation and/or infection is the pretreatment endodontic and periradicular diagnosis. If the treatment diagnosis is irreversible pulpitis with or without symptomatic apical periodontitis, this is strictly inflammation and anti-inflammatory agents — nonsteroidal anti-inflammatory drugs (NSAIDs), for example — are the medication of choice.
A significant reduction in odontogenic pain from inflammation can be seen from taking 400 to 800 mg of ibuprofen.22 A recent study by Tagger et al23 found that ibuprofen sodium dihydrate provided faster pain relief than ibuprofen acid. In cases in which ibuprofen is not effective in reducing postoperative pain for an endodontic patient, administering a combination of ibuprofen and acetaminophen can help manage pain from odontogenic-type inflammation.24 Acetaminophen alone or in combination with an opioid (e.g., hydrocodone) is a good alternative analgesic in patients who cannot take NSAIDs.6
There will be cases in which NSAIDs do not relieve a patient’s odontogenic postoperative pain. Although opioids are commonly prescribed in these scenarios, clinicians should also consider prescribing dexamethasone, a synthetic adrenocortical steroid.25 If there is an odontogenic infection, a patient should be placed on an antibiotic, too, because steroids can block or mask the body’s response to infection. When prescribing dexamethasone, due to the increased risk of developing stomach problems (such as a bleeding stomach ulcer), patients should be taken off NSAIDs if they are currently taking them.
The trend in prescribing antibiotics is as adjunctive treatment to conventional or surgical endodontic therapy. This adjunctive antibiotic treatment may be necessary to prevent the spread of infection, in acute apical abscesses with systemic involvement, and in progressive and persistent infections.26 Systemic involvement in clinical infection can appear as fever, swelling, malaise, compromised airway, cellulitis and/or a medically compromised patient.
Penicillin V potassium (Pen VK) has been documented in the literature as the antibiotic of choice for endodontic infections.27 It has been demonstrated that its spectrum of antimicrobial activity includes many of the bacteria that have been isolated in endodontic infections.28 Segura-Egea et al26 report that amoxicillin alone or with clavulanic acid shows better absorption and tissue penetration, as well as fewer side effects than Pen VK; these agents also have a wider spectrum of activity than Pen VK. This includes many species of bacteria found elsewhere in the body and may increase the risk of selecting for bacterial resistance outside of the oral cavity. However, amoxicillin and clavulanic acid are indicated for the treatment of immunocompromised patients who may have odontogenic infections containing nonoral bacteria.28
For patients who are allergic to penicillin/amoxicillin or if penicillin/ amoxicillin has been ineffective, clindamycin is the second antibiotic of choice. Clindamycin is beta-lactamase resistant (unlike Pen VK) and has a good spectrum against gram-positive and gram-negative bacteria.29 Another option if Pen VK is ineffective is to add metronidazole along with Pen VK. Due to the fact that metronidazole has a narrow therapeutic spectrum against obligate anaerobic bacteria,30 it should not be prescribed as the sole antibiotic; rather, it should be used in combination with Pen VK.
Another important consideration when prescribing antibiotics is to use a loading dose. This is because antibiotics with long half-lives can require several days of therapy to take full effect. The most critical time for antibiotic efficacy is the first 24 hours, as this is typically when inoculum of infection is high and likely to harbor resistant subpopulations of bacteria.31,32 An antibiotic regimen in endodontic therapy should extend seven to 10 days. Although a patient should respond to antibiotic therapy within 24 to 48 hours, the individual needs to stay on the antibiotic until finished to prevent a rebound effect of the active infection. In addition, the clinician should be in close contact with a patient on antibiotics in case clinical symptoms worsen or there is a drug allergy.33 Finally, if an endodontic patient presents with intraoral fluctuant swelling, the clinician should perform an incision-and-drain procedure.34
SUMMARY
Appropriate pain management in endodontic therapy requires clinicians to have a good understanding of local anesthesia, conventional endodontic treatment and clinical pharmacology. Equipped with these clinical skills, the dentist or specialist will be positioned to successfully manage the pain reported by their endodontic patient.
REFERENCES
- Cohen HP, Cha BY, Spångberg LS. Endodontic anesthesia in mandibular molars: a clinical study. J Endod. 1993;19:370–373.
- Nusstein J, Reader A, Nist R, Beck M, Meyers WJ. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1:100,000 epinephrine in irreversible pulpitis. J Endod. 1998;24:487–491.
- Fuss Z, Trowbridge H, Bender IB, Rickoff B, Sorin S. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12:301–305.
- Hargreaves KM, Keiser K. New advances in the management of endodontic pain emergencies. J Calif Dent Assoc. 2004;32:469–473.
- Hasselgren G, Reit C. Emergency pulpotomy: pain relieving effect with and without the use of sedative dressings. J Endod. 1989;15:254–256.
- Hargreaves KM. Management of pain in endodontic patients. Tex Dent J. 1997;114:27–31.
- Ricucci D, Loghin S, Siqueira J. Correlation between clinical and histological diagnosis. J Endod. 2014;40:1932–1939.
- Taha NA, Ahmad MB, Ghanim A. Assessment of mineral trioxide aggregate pulpotomy in mature permanent teeth with carious exposures. Int Endod J. 2017;50:117–125.
- Hilton TJ, Ferracane JL, Manci L, Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res. 2013;92(suppl 7):16s–22s.
- Wang Z. Bioceramic materials in endodontics. Endod Topics. 2015;32:3–30.
- Rosenberg PA, Babick PJ, Schertzer L, Leung A. The effect of occlusal reduction on pain after endodontic instrumentation. J Endod. 1998;24:492–496.
- Yu DC, Tam A, Schilder H. Root canal anatomy illustrated by microcomputed tomography and clinical cases. Gen Dent. 2006;54:331–335.
- Guelzow A, Stamm O, Martus P, Kielbassa AM. Comparative study of six rotary nickel-titanium systems and hand instrumentation for root canal preparation. Int Endod J. 2005;38:743–752.
- Gernhardt CR, Eppendorf A, Kozlowski A, Brandt M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004;37:272–280.
- Singh RD, Khatter R, Bal RK, Bal CS. Intracanal medication versus placebo in reducing postoperative endodontic pain — a double-blind randomized clinical trial. Braz Dent J. 2013;24:25–29.
- Walton R, Fouad A. Endodontic inter-appointment flare-ups: a prospective study of incidence and related factors. J Endod. 1992;18:172–177.
- Siqueira JF. Microbial causes of endodontic flare-ups. Int Endod J. 2003;36:452–463.
- Yoldas O, Topuz A, Isçi AS, Oztunc H. Postoperative pain after endodontic retreatment: single-versus two-visit treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:483–487.
- Mattscheck DJ, Law AS, Noblett WC. Retreatment versus initial root canal treatment: factors affecting posttreatment pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:321–324.
- Alí A, Olivieri JG, Duran-Sindreu F, Abella F, Roig M, García-Font M. Influence of preoperative pain intensity on postoperative pain after root canal treatment: A prospective clinical study. J Dent. 2016;45:39–42.
- Fouad AF, Rivera EM, Walton RE. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:590–595.
- Torabinejad M. Cymerman JJ, Frankson M, Lemon RR, Maggio JD, Schilder H. Effectiveness of various medications on postoperative pain following complete instrumentation. J Endod. 1994;20:345–354.
- Tagger T, Wu D, Khan A. A randomized clinical trial comparing 2 ibuprofen formulations in patients with acute odontogenic pain. J Endod. 2017;43:674–678.
- Cooper SA. The relative efficacy of ibuprofen in dental pain. Compend Contin Educ Dent. 1986;7:578,580–581.
- Krasner P, Jackson E. Management of posttreatment endodontic pain with oral dexamethasone: a double-blind study. Oral Surg Oral Med Oral Pathol. 1986;62:187–190.
- Segura-Egea JJ, Gould K, Sen BH, et al. Antibiotics in endodontics: a review. Int Endod J. December 22, 2016. Epub ahead of print.
- Kuriyama T, Karasawa K, Nakagawa K, Saiki Y, Yamamoto E, Nakamura S. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Rad Endod. 2000;90:600–608.
- Baumgartner JC, Xia T. Antibiotic susceptibilty of bacteria associated with endodontic abscesses. J Endod. 2003;29:44–47.
- Gilmore WC, Jacobus NV, Gorbach SL, Doku HC, Tally FP. A prospective double-blind evaluation of penicillin versus clindamycin in the treatment of odontogenic infections. J Oral Maxillofac Surg. 1988;46:1065–1070.
- Sandor G, Low DE, Judd PL, Davidson RJ. Antimicrobial treatment options in the management of odontogenic infections. J Can Dent Assoc. 1998;64:508–514.
- Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat Rev Microbiol. 2004;2:289–300.
- Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetics/pharmacodynamics target. Antimicrob Agents Chemother. 2012;56:2795–2805.
- Bahcall JK. Everything I know about endodontics, I learned after dental school, Part 2. Dent Today. 2003;22:62–68.
- Newman M, Kornman K. Antibiotics/Antimicrobial Use in Dental Practice. Chicago, Ill: Quintessence;1984:152.
Featured Image by DUSANMANIC/ISTOCK/GETTY IMAGES PLUS
From Decisions in Dentistry. August 2017;3(8):45–49.




