
Long-Term Effects of Head and Neck Radiation on Oral Health
Because patients undergoing radiotherapy for head and neck cancer are susceptible to complications affecting tissues of the oral cavity, a comprehensive understanding of side effects is pivotal to successful case management.
This course was published in the May 2018 issue and expires May 2021. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the prevalence of head and neck cancer, as well as the estimated percentage of patients who will undergo radiation therapy and develop resulting oral complications.
- Discuss the categorization of head and neck cancer, and common approaches to treatment.
- List the oral complications of head and neck cancer therapy, and clinical management strategies for oral health professionals.
Introduction
As health care professionals, it is important to take into account the special needs of patients undergoing radiation for head and neck cancer, as these individuals typically require extra care, knowledge and fluoride to help counter the oral side effects of therapy. The American Dental Association notes that high-risk patients can benefit from additional sources of fluoride, and one option is substituting a regular fluoride toothpaste with a prescription-strength dentifrice containing 5000-ppm fluoride. In “Long-Term Effects of Head and Neck Radiation on Oral Health,” the authors provide a detailed review of oral health complications from radiotherapy, including oral mucositis, xerostomia, gland dysfunction, soft tissue necrosis, osteoradionecrosis and oral candidiasis. This insightful article also outlines key steps for effective care, and the importance of periodontal treatment before and after radiation.
I hope you will find this paper to be a valuable resource when caring for these patients and the anticipated oral complications of radiation treatment. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this continuing education article and series.
—Matilde Hernandez, DDS, MS, MBA
Scientific Affairs Manager Professional Oral Care
Colgate Oral Pharmaceuticals
The Oral health consequences of radiation therapy for head and neck cancer require a collaborative approach to treatment
From the American Academy of Periodontology
Radiation therapy is a common treatment for various forms of cancer. However, just as radiation shrinks tumors and destroys cancer cells, healthy tissue near the targeted area is also susceptible to damage. For individuals with head and neck cancers, radiation may result in gingival recession and loss of alveolar bone — key features of periodontal disease.
In this article, educators and American Academy of Periodontology (AAP) members Andrea Ravidà, DDS, MS, and Fernando Suárez López del Amo, DDS, MS, highlight the effect radiation has on the oral cavity, and how periodontal treatment can help patients and practitioners manage the potential side effects. In addition to a patient’s self-care maintenance, the role of oral health professionals is key in minimizing oral complications that may arise from cancer care.
The AAP is proud to work with Decisions in Dentistry and Colgate-Palmolive to help prepare dentists, dental hygienists and dental assistants as key partners in the collaborative management of patients whose medical conditions impact their oral health.
—Steven R. Daniel, DDS
President, American Academy of Periodontology
Cancer is the second most common cause of death in the United States, accounting for 25% of all fatalities. More than 1.6 million new cancer cases were expected to be diagnosed in 2017, and approximately 600,920 patients were expected to die. This translates to 1650 deaths per day in the U.S. alone.1 In Europe, cancer incidence is significantly higher, but this discrepancy is buffered when incidence per population is taken into account. The prevalence of cancer in Europe was more than 3.7 million cases in 2012, where 1.9 million patients were expected to die of the disease.2,3 It has also been shown that cancer incidence rates vary by gender, race, ethnicity and age.

Among men, the most common worldwide form is lung cancer, which made up nearly 17% of the new cancer cases diagnosed in 2012. Among women, breast cancer represented more than 25% of new cases diagnosed in 2012.2 Not far behind, head and neck cancer collectively ranks 8th in terms of estimated new cases. A projected 49,670 new cases in the oral cavity and pharynx will be diagnosed in the U.S. in 2017, resulting in an estimated 9700 deaths. Of these patients, incidence rates are more than twice as high for men than women.1,4 Head and neck cancer is a communal subset of tumors that is categorized by the area in which it initiates. These include the oral cavity (including lips, tongue, gingiva, cheeks, lips, floor of the mouth and palate); pharynx (including nasopharynx, oropharynx, base of the tongue and hypopharynx); larynx; paranasal sinuses and nasal cavity; and the salivary glands.
Several approaches might be used to treat head and neck cancer, including surgical procedures, radiation therapy (RT), chemotherapy, targeted therapy, or combination of these modalities. The choice of treatment depends on multiple factors, including location of the tumor, stage of cancer, general health and age.5,6
RADIATION THERAPY
Typically, more than 50% of newly diagnosed cancer patients will receive RT as part of a combined modality treatment that includes either surgery or chemotherapy.7 Delivered by an external- or internal-beam therapy protocol, RT shrinks tumors and kills cancer cells by damaging their DNA. All cancer cells with DNA damaged beyond the cell’s ability to repair will eventually die or stop dividing.8 A key limitation is RT’s propensity to damage healthy cells near the treated area. By virtue of its rapid cell turnover, the lining of oral mucosa is a main target for radiation-related toxicity.9 The oral complications resulting from head and neck RT can be divided into two groups.10 Acute complications occurring during therapy include oral mucositis; xerostomia; infections (predominately candidiasis); and taste dysfunction. After RT is complete, chronic complications may arise, including mucosal fibrosis and atrophy; decreased salivary secretion and xerostomia; accelerated caries associated with compromised salivary secretion; infections (primarily candidiasis); tissue necrosis (soft tissue necrosis and osteonecrosis); taste dysfunction (dysgeusia/ageusia); muscular and cutaneous fibrosis; and dysphagia.
ORAL MUCOSITIS
Due to the rapid epithelial turnover of the mucosa, oral mucositis is one of the earliest and most common side effects following head and neck RT. At least 80% of patients receiving RT will experience oral mucositis.11 This acute response occurs during the first two to three weeks of treatment, resulting in inflammation of oral mucosa (usually manifesting as ulceration) and exudate formation or erythema, which might be subsequently covered by a pseudomembrane.12,13 The advent of such a painful condition interferes with the patient’s ability to function, thus impacting tolerance for continued cancer therapy.11,14
A comprehensive assessment of the oral cavity following RT ensures early identification of oral lesions. Oral hygiene and other supportive care measures are important to reducing lesion severity.15 These include cleaning the oral mucosa, lubrication of lips and oral tissues, and maintaining hydration, as well as palliative measures.
In order to standardize measurements of mucosal integrity in clinical practice and research, the severity of oral mucositis should be evaluated using accepted scales, such as the World Health Organization (WHO) mucositis scale16 (Table 1) and the Common Terminology Criteria for Adverse Events (CTCAE; Table 2).17 Combining subjective and objective measures, the WHO scale is easy to use in daily practice. Introduced by the National Cancer Institute, CTCAE version 3.0 includes subjective and objective scales for mucositis, and is often used to measure overall toxicity. Based on these scales, 62% of Grade 3 and Grade 4 mucositis cases will require hospitalization, and 70% will require feeding tubes.13 Several professional organizations have produced evidence-based guidelines for managing oral mucositis. The Cochrane Collaboration uses a meta-analysis approach, and thus provides a unique context for guidelines based on the aforementioned scales.18,19
In 2004, the U.S. Food and Drug Administration (FDA) approved a recombinant protein called palifermin that, when used intravenously, decreases the incidence and duration of severe oral mucositis.20 Evidence from other studies supported low-level laser therapy to prevent oral mucositis in patients with head and neck cancer who were undergoing RT, without concomitant chemotherapy.21,22
In a systematic review, McGuire et al23 investigated additional interventions, including oral care protocols, dental care, saline, sodium bicarbonate, mixed medication mouthrinse, chlorhexidine and calcium phosphate. The authors concluded that no recommendations in favor of a particular intervention could be suggested due to inadequate evidence. Instead, plain water can be used — an approach that is typically tolerated by patients and promotes observance of basic oral hygiene practices.23
XEROSTOMIA AND SALIVARY GLAND DYSFUNCTION
Salivary gland hypofunction and xerostomia are among the most common and severe late complications of head and neck RT. Ionizing radiation to minor and major salivary glands results in inflammatory and degenerative consequences to salivary gland parenchyma; more specifically, loss of acinar cells, alteration in duct epithelium, fibrosis and fatty degeneration. The early salivary gland tissue response to RT results in salivary gland hypofunction (objective evidence of decreased salivary flow) within the first week of treatment, and xerostomia (the subjective feeling of dry mouth), which becomes apparent when administered doses exceed 10 Gy.24
Symptoms of salivary gland hypofunction include xerostomia, lip dryness and crusting, fissures at lip commissures, and an oral burning sensation. They can also include altered taste and difficulties with speech, chewing, swallowing or wearing dentures. These adverse effects, which range from mild to severe, typically impact a patient’s quality of life following radiation treatment.25 In addition, it is unlikely that salivary gland function will recover completely after radiation. As a result, unless preventive measures are instituted, periodontal disease can be accelerated and caries can become rampant.
Preventive strategies designed to maintain optimal oral hygiene will minimize the risk of oral lesions. Suggested local measures include brushing with fluoridated dentifrice and using a therapeutic rinse at least four times per day, as well as regular topical application of fluoride. Prevention can also be approached through newer modalities, such as intensity-modulated radiation therapy26 and intravenous amifostine,27 although results from both modalities require support from further studies.
Treatment of radiation-induced salivary gland hypofunction and xerostomia is primarily symptomatic, for example, sipping or spraying the oral cavity with water, using saliva substitutes (e.g., oral rinses or gels containing hydroxyethylcellulose), or stimulation of saliva production from salivary glandular tissues by taste, mastication, pharmacological sialogogues or acupuncture. Sugar-free lozenges and chewing gum can also provide transient relief from xerostomia by stimulating the residual competence of salivary gland tissue.26 Pilocarpine is the only drug approved by the FDA as a sialogogue for radiation-induced xerostomia. However, it was suggested that its efficacy is dose related (≥ 40 Gy).28 In addition, one of the more common adverse effects when using pilocarpine is hyperhidrosis; however, its incidence and severity are typically proportional to dosage. Other adverse effects are expected at even higher doses (≥ 5 mg three times per day) of pilocarpine. These include nausea, vasodilation, headache, rhinorrhea, increased lacrimation, urinary urgency and frequency, asthenia, diarrhea and dyspepsia.29,30 Another approach that offers valid intervention in these patients is acupuncture, which seems to enhance the residual functional capacity of the salivary glands without serious adverse effects.31,32

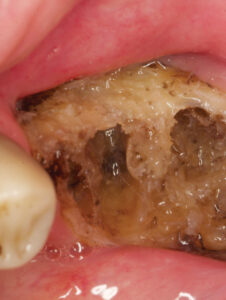
OSTEORADIONECROSIS
A long-standing, nonhealing area(s) of exposed bone in patients who have been treated with RT, osteoradionecrosis (ORN) is one of the most serious complications in this patient population (Figure 1 and Figure 2). Associated with pain, infection, fistula formation, diminished or complete loss of sensation, and morbidity, advanced stages of ORN will typically require management via surgical resection and reconstruction.21 Risk directly relates to radiation dose and the volume of tissue irradiated. Unlike the maxilla, each side of the mandible has a unilateral blood supply, which explains why ORN most frequently manifests in the mandible. Since bone turnover and repair is compromised in ORN-involved sites, pathologic fracture can occur because the bone is unable to repair itself.33,34 Management is chiefly based on prevention, and begins with comprehensive oral assessment and care prior to RT. During the pretreatment phase, all oral diseases should be treated, any dentition with poor prognosis within high-dose fields should be extracted, and any source of trauma from prostheses should be modified or eliminated.21
As for treatment, topical antibiotics or antiseptics may contribute to wound resolution, and analgesics are indicated for pain control. If a surgical approach is attempted, local resection of bone sequestra and soft tissue coverage of the exposed bone should be achieved. Hyperbaric oxygen therapy (HBO) increases the oxygenation of irradiated tissue and promotes angiogenesis. If used prior to extractions, it has proven to decrease the incidence of ORN in head and neck irradiated patients.35 However, cost and the lack of accessibility to HBO technology are limiting factors.36 A systematic review of treatment strategies concluded that HBO can actively contribute in the management of ORN, yet no clear recommendation for its use in the prevention or treatment of ORN was established.21
ORAL CANDIDIASIS
Classically caused by opportunistic overgrowth of Candida albicans, several variables contribute to the clinical expression of oral candidiasis, including drug- or disease-induced immunosuppression and decreased salivary outflow — which are common in this patient population. Regular use of antibiotics may also alter the balance of oral flora, creating a favorable environment for fungal overgrowth.12 Forms of oral candidiasis most commonly reported in patients with head and neck cancer are pseudomembranous and erythematous.37,38 Pseudomembranous candidiasis is diagnosed on the basis of its clinical appearance, and is occasionally accompanied by altered taste sensation and burning pain. Erythematous candidiasis may also be accompanied by a burning sensation, although its clinical presentation is relatively nonspecific and laboratory investigations are usually needed to confirm a diagnosis.31,39
Daily oral hygiene measures should be maintained to decrease the incidence and severity of oral candidiasis. Nystatin rinses and clotrimazole troches are two common topical antifungal agents that are used orally. Patients with removable dentures should remove them before the oral antifungal agents are applied and soak the dentures overnight in the antifungal solution. Systemic agents are usually reserved for more persistent fungal infections and patients with significant immunosuppression. Systemic fluconazole is highly effective for prophylaxis and treatment of oral fungal infections in this patient population.12

SOFT TISSUE NECROSIS
Soft tissue necrosis of previously irradiated tissue is another serious complication in patients who have undergone RT for head and neck tumors. Acute effects typically involve the oral mucosa, and chronic changes are usually a result of vascular inflammation and scarring that result in hypoxic, hypovascular and hypocellular changes. Necrosis can involve mucosal surfaces in the oral cavity. The highest risk usually involves nonkeratinized tissues. Soft tissue necrosis begins as an ulcerative breakdown in the surface of oral mucosa and can spread in diameter and depth. If necrosis progresses, pain will likely become prominent. If infection occurs secondary to hard tissue injury and osteonecrosis, tissue necrosis will be confounded even more.40,41 Trauma and injury are often associated with nonhealing soft tissue necrotic lesions, although spontaneous lesions have also been noted.40
PERIODONTAL DISEASE
Loss of alveolar bone is the hallmark of periodontitis, and disease severity can be categorized on basis of clinical attachment loss of supporting structures.42 In many cases, periodontitis results in pain, infection of the periapical alveolar bone, and tooth loss.43 Compared to the general population, patients with head and neck cancer treated with RT are at higher risk for periodontal disease, with periodontitis potentially prompting ORN. Periodontitis will typically worsen with cancer treatment; nevertheless, failure to treat periodontitis may eventually lead to tooth extraction, which may, in turn, contribute to the development of ORN.33
Deterioration of periodontal health occurs once RT has started and is typically dose-dependent.44 This is concomitant with reduced vascularity and cellularity of the periodontal ligaments, as well as rupturing, thickening and disorientation of Sharpey’s fibers, and widening of periodontal ligaments following RT.45 In a study comparing pre- and post-RT patients, the key features noted in the latter included significant, rapid loss of supporting periodontal structures, including gingival recession and alveolar bone loss.46 In this population, RT typically leads to reduced salivary flow, which reduces the protective effects saliva and causes shifts in the oral microbiome toward periodontal disease-associated flora.47,48 In the absence of good oral hygiene, rampant periodontal destruction is expected. Current recommendations state that head and neck cancer patients be examined and treated by a dentist who is aware of the planned cancer therapy and anticipated oral complications before, during and following therapy.49
Periodontal treatment prior to RADIATION therapy
The periodontium should be thoroughly examined prior to initiating RT to identify disease that could lead to serious periodontal infection. Periodontal disease is a primary cause of tooth loss in adults. Consequently, teeth with advanced attachment loss or those anticipated to require future surgical management within an area of planned high-dose RT should be extracted prior to RT. Comprehensive dental and periodontal examinations are required to identify these teeth.50 In addition, a commitment to preventive oral health management is needed to reduce risks of further periodontal involvement, which may lead to tooth extraction — with consequent risk of ORN.50
Recommended clinical and radiographic examinations include restoration of all active caries lesions, extraction of all nonrestorable teeth (including any with advanced periodontal disease), root canal treatment, or extraction of all nonvital teeth and those with apical lesions.51,52 If partially erupted, third molars should also be extracted before RT due to the risk of complications; this also applies if management of the third molar will interfere with cancer therapy.52
Use of local and topical antiseptic agents has shown promising results in controlling periodontal health. One study compared the effect of 0.12% chlorhexidine, 0.5% sodium fluoride, 2% sodium iodine and no intervention starting three to four weeks prior to head and neck RT. When compared to no intervention, the authors noted a significant reduction in plaque index at six months in all three intervention groups.53 Moreover, compared to the other two interventions, the chlorhexidine group saw the biggest decrease in salivary Streptococcus mutans counts.53 It should also be noted that progression of periodontal disease in patients with advanced stages of cancer is aggravated by the indirect (i.e., physiological) effect of the cancer.54 Patients often develop physical problems, such as chronic fatigue and nausea, that may hamper their ability to perform oral hygiene. Additionally, patients often develop psychological problems (depression, for example) and/or cognitive issues, which may further affect their ability or desire to undertake oral hygiene measures.55
Periodontal Care After Radiation
Supportive periodontal therapy and home care are essential to maintaining post-RT periodontal health. Fluoride therapy and chlorhexidine mouthrinse have proven particularly beneficial for these patients.49 Therefore, a rigorous protocol for post-radiation compliance with supportive periodontal therapy should be followed with patients who survive head and neck cancer.
Conclusion
Patients undergoing radiotherapy for head and neck cancer are susceptible to side effects that manifest in various tissues of the oral cavity. Thus, collaboration between medical and dental teams is pivotal to successful case management. Because RT can result in oral mucositis, salivary gland dysfunction, osteoradionecrosis, soft tissue necrosis, oral candidiasis and periodontal disease, risk management and prevention become the primary tools for management. With these goals in mind, it is advisable to extract teeth with a poor prognosis before starting radiotherapy. Each tooth maintained by the dental team must be carefully examined for pathologic conditions and restored to the best state of health possible. A thorough prophylaxis, along with chlorhexidine mouthrinse and topical fluoride, should be utilized before, during and after radiotherapy. While the importance of effective self-care cannot be overemphasized, clinicians should also consider that patients with head and neck cancer often develop physical and/or psychological problems that compromise their ability (or desire) to perform oral hygiene. In light of the foregoing, this patient population is best served by providers who are aware of the planned cancer therapy and anticipated oral complications.49
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30.
- Ferlay J, Ervik M, Dikshit R, et al. World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at: globocan.iarc.fr. Accessed April 13, 2018.
- Forman D, Bray F, Brewster C, et al. Cancer incidence in five continents vol. X. IARC Publications. Available at: iarc.fr/en/publications/pdfs-online/epi/sp164/CI5volX_Full.pdf. Accessed April 13, 2018.
- Komatsubara KM, Carvajal RD. The promise and challenges of rare cancer research. Lancet Oncol. 2016;17:136–138.
- Hashibe M, Boffetta P, Zaridze D, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006;15:696–703.
- American Cancer Society. Cancer Facts and Figures 2017. Available at: cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017. Accessed April 13, 2018.
- Chang AE, Ganz PA, Hayes DF, et al, eds. Oncology: An Evidence-Based Approach. New York, NY: Springer; 2006.
- DeVita V Jr, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2008.
- Yagiela JA, Dowd FJ, Johnson BS, et al, eds. Pharmacology and Therapeutics for Dentistry. 6th ed. St. Louis: Mosby; 2011:782.
- Brennan MT, Elting LS, Spijkervet FK. Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: methodology and quality of the literature. Support Care Cancer. 2010;18:979–984.
- Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–39.
- Lalla RV, Latortue MC, Hong CH, et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer. 2010;18:985–992.
- Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025.
- Borbasi S, Cameron K, Quested B, Olver I, To B, Evans D. More than a sore mouth: patients’ experience of oral mucositis. Oncol Nurs Forum. 2002;29:1051–1057.
- Larson PJ, Miaskowski C, MacPhail L, et al. The PRO-SELF Mouth Aware program: an effective approach for reducing chemotherapy-induced mucositis. Cancer Nurs. 1998;21:263–268.
- World Health Organization. Handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979:15. Available at: http://apps.who.int/iris/bitstream/handle/10665/37200/WHO_OFFSET_48.pdf?sequence=1&isAllowed=y. Accessed April 13, 2018.
- Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer. 1992;69:2469–2477.
- Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010:CD000978.
- Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010:CD001973.
- Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–2820.
- Peterson DE, Doerr W, Hovan A, et al. Osteoradionecrosis in cancer patients: the evidence base for treatment-dependent frequency, current management strategies, and future studies. Support Care Cancer. 2010;18:1089–1098.
- Kuhn A, Porto FA, Miraglia P, Brunetto AL. Low-level infrared laser therapy in chemotherapy-induced oral mucositis: a randomized placebo-controlled trial in children. J Pediatr Hematol Oncol. 2009;31:33–37.
- McGuire DB, Fulton JS, Park J, et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:3165–3177.
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212.
- Teguh DN, Levendag PC, Voet P, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008;30:622–630.
- Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039–1060.
- Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, de Vries A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64:684–691.
- Burlage FR, Roesink JM, Kampinga HH, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys. 2008;70:14–22.
- Rieke JW, Hafermann MD, Johnson JT, et al. Oral pilocarpine for radiation-induced xerostomia: integrated efficacy and safety results from two prospective randomized clinical trials. Int J Radiat Oncol Biol Phys. 1995;31:661–669.
- Scarantino C, LeVeque F, Swann RS, et al. Effect of pilocarpine during radiation therapy: results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J Support Oncol. 2006;4:252–258.
- Blom M, Lundeberg T. Long-term follow-up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Dis. 2000;6:15–24.
- Cho JH, Chung WK, Kang W, Choi SM, Cho CK, Son CG. Manual acupuncture improved quality of life in cancer patients with radiation-induced xerostomia. J Altern Complement Med. 2008;14:523–526.
- Katsura K, Sasai K, Sato K, Saito M, Hoshina H, Hayashi T. Relationship between oral health status and development of osteoradionecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:731–738.
- Reuther T, Schuster T, Mende U, Kübler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients —a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295.
- Sulaiman F, Huryn JM, Zlotolow IM. Dental extractions in the irradiated head and neck patient: a retrospective analysis of Memorial Sloan-Kettering Cancer Center protocols, criteria, and end results. J Oral Maxillofac Surg. 2003;61:1123–1131.
- Adkinson C, Anderson T, Chavez J, et al. Hyperbaric oxygen therapy: a meeting place for medicine and dentistry. Minn Med. 2005;88:42–45.
- Nicolatou-Galitis O, Athanassiadou P, Kouloulias V, et al. Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer. 2006;14:753–762.
- Nicolatou-Galitis O, Velegraki A, Sotiropoulou-Lontou A, et al. Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support Care Cancer. 2006;14:44–51.
- Jham BC, Teixeira IV, Aboud CG, Carvalho AL, Coelho Mde M, Freire AR. A randomized phase III prospective trial of bethanechol to prevent radiotherapy-induced salivary gland damage in patients with head and neck cancer. Oral Oncol. 2007;43:137–142.
- National Cancer Institute. Oral complications of chemotherapy and head/neck radiation: Health Professional Version; 2017. Available at: cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq. Accessed April 13, 2018.
- Silverman S Jr. Oral Cancer. 5th ed. Hamilton, ON: Canada: BC Decker Inc; 2003:113.
- American Academy of Periodontology Task Force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86:835–838.
- Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918–2931.
- Hommez GM, De Meerleer GO, De Neve WJ, De Moor RJ. Effect of radiation dose on the prevalence of apical periodontitis — a dosimetric analysis. Clin Oral Investig. 2012;16:1543–1547.
- Silverman S, Jr., Chierici G. Radiation therapy of oral carcinoma. I. Effects on oral tissues and management of the periodontium. J Periodontol. 1965;36:478–484.
- Ammajan RR, Joseph R, Rajeev R, Choudhary K, Vidhyadharan K. Assessment of periodontal changes in patients undergoing radiotherapy for head and neck malignancy: a hospital-based study. J Cancer Res Ther. 2013;9:630–637.
- Al-Nawas B, Grötz KA. Prospective study of the long term change of the oral flora after radiation therapy. Support Care Cancer. 2006;14:291–296.
- Laheij AM, de Soet JJ. Can the oral microflora affect oral ulcerative mucositis? Curr Opin Support Palliat Care. 2014;8:180–187.
- Hong CH, Napenas JJ, Hodgson BD, et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer. 2010;18:1007–1021.
- Hong CHL, Hu S, Haverman T, et al. A systematic review of dental disease management in cancer patients. Support Care Cancer. 2018;26:155–174.
- Melkos AB, Massenkeil G, Arnold R, Reichart PA. Dental treatment prior to stem cell transplantation and its influence on the posttransplantation outcome. Clin Oral Investig. 2003;7:113–115.
- Tai CC, Precious DS, Wood RE. Prophylactic extraction of third molars in cancer patients. Oral Surg Oral Med Oral Pathol. 1994;78:151–155.
- Meca LB, Souza FR, Tanimoto HM, Castro AL, Gaetti-Jardim Junior E. Influence of preventive dental treatment on mutans streptococci counts in patients undergoing head and neck radiotherapy. J Appl Oral Sci. 2009;17(Suppl):5–12.
- Davies A, Finlay I. Oral Care in Advanced Disease. Oxford: Oxford University Press; 2005.
- Davies A, Epstein B. Oral Complications of Cancer and its Management. Oxford: Oxford University Press; 2010.
Featured image Courtesy PABLO GALINDO-MORENO, DDS, PhD
From Decisions in Dentistry. May 2018;4(5):37-42.




